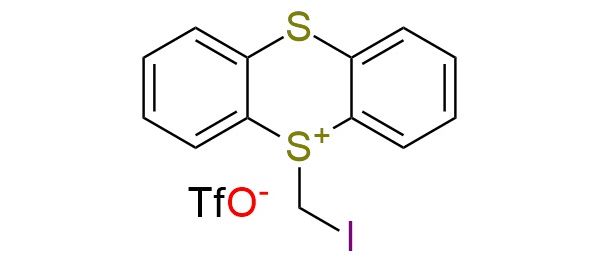
CAS 3095012-65-7, Cat. No EN300-54097048
Reagent for cyclization and alkenes functionalization
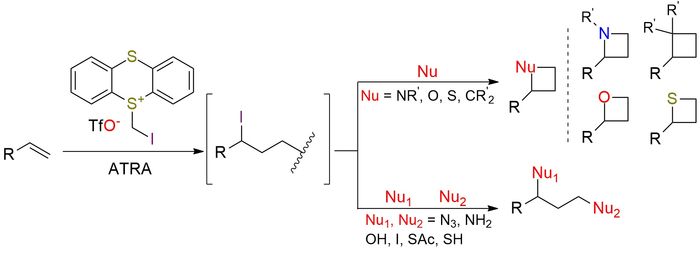
5-(Iodomethyl)-5H-thianthrenium triflate (TT+-CH2I TfO-) serves as a dual electrophilic (dielectrophilic) synthon, enabling the conversion of unactivated alkenes into highly reactive iodo–sulfonium intermediates under visible-light photoredox conditions1. Upon photocatalytic atom-transfer radical addition (ATRA), it generates a transient 1,3-bis-electrophilic species bearing both an alkyl iodide and a sulfonium center. The distinct reactivity of the intermediate allows sequential nucleophilic substitution to form 1,3-difunctionalized products such as diazides, diamines, diols, dihalides, dithiols, and a broad range of heterodifunctionalized derivatives. There is a methodology2 for synthesizing four-membered rings, including azetidines, oxetanes, thietanes, and cyclobutanes, directly from alkenes. The method enables selective transformations without the need for transition-metal catalysis, demonstrating good compatibility with common functional groups. The reagent exhibits exceptional functional-group tolerance, accommodating a wide range of compounds, including esters, amides, nitriles, alcohols, amines, sulfides, sulfones, phosphonates, boronic esters, and heteroaromatics. By tuning the nucleophile and reaction conditions (including the use of silver salts or bases), the order of substitution can be controlled, enabling selective access to different ring classes.
Synonyms: thianthrenium, 5-(iodomethyl)-, 1,1,1-trifluoromethanesulfonate; 5-(iodomethyl)-5H-thianthren-5-ium trifluoromethanesulfonate; 5-(Iodomethyl)-5H-thianthren-5-ium triflate; iodomethyl thianthrenium triflate; TT+-CH2I TfO-
Selected publications
-
Thianthrenium Salt‐Mediated Homologative 1,3‐Dielectrophilic Activation Strategy for Alkene Difunctionalization.
Paul, S.; Richardson, A.; Buck, L.; Centonze, G.; Coccia, R.; Sardelli, F.; Silvi, M. Angewandte Chemie Novit 2025, 1 (1). DOI: 10.1002/anov.70005
-
A Unified Synthetic Approach to 2-Alkyl Azetidines, Oxetanes, Thietanes and Cyclobutanes from Unactivated Alkenes.
Buck, L.; Pelosi, M.; Paul, S.; Wheatley, E.; Richardson, A.; Ghosh, S.; Sardelli, F.; Silvi, M. J Am Chem Soc 2025, 147 (49), 44652–44660. DOI: 10.1021/jacs.5c11758