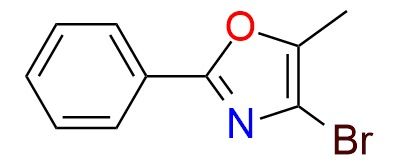
CAS 197719-26-9, Cat. No EN300-22892964
Cyanide-free hydrocyanation reagent
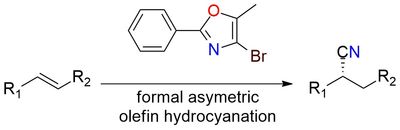
4-Bromo-5-methyl-2-phenyl oxazole is a cyanide-free hydrocyanation reagent used in Pd/CuH-catalyzed asymmetric olefin hydrocyanation1. It serves as a masked nitrile equivalent, undergoing hydroarylation followed by [4+2]/retro-[4+2] cycloaddition to generate enantioenriched nitriles2. This method offers a safe alternative to hazardous hydrogen cyanide, making it valuable for pharmaceutical synthesis. The reagent enables selective Markovnikov and anti-Markovnikov hydrocyanation, producing bioactive nitrile-containing molecules. It is a bench-stable solid, ensuring controlled cyanation under mild conditions. The reagent scalability and selectivity make it a practical tool for medicinal and industrial chemistry.
Synonyms: 4-bromo-5-methyl-2-phenyl-1,3-oxazolel; 2-bromo-4-phenyl-1,3-oxazole
Selected publications
-
Enantioselective Olefin Hydrocyanation without Cyanide.
Schuppe A. W.; Borrajo-Calleja G. M.; Buchwald S. L. J Am Chem Soc 2019, 141 (47), 18668–18672. DOI: 10.1021/jacs.9b10875
-
Cyanide‐Free Cyanation of sp2 and sp‐Carbon Atoms by an Oxazole‐Based Masked CN Source Using Flow Microreactors.
Sharma B. M.; Nikam A. V.; Lahore S.; Ahn G.; Kim D. Chemistry – A European Journal 2022, 28 (20). DOI: 10.1002/chem.202103777