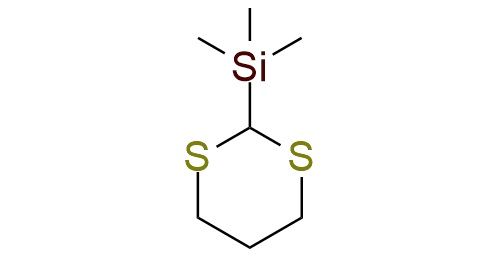
CAS 13411-42-2, Cat. No EN300-7646366
Reagent for dithioketene acetals synthesis
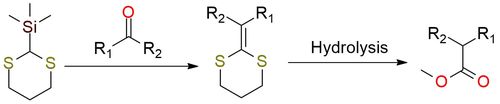
2-Trimethylsilyl-1,3-dithiane is a reagent precursor to many dithioketene acetals1. This reaction is the first two-step procedure that results in the one-carbon homologation of an aldehyde or ketone via a Peterson olefination. The following dithiane hydrolysis gives the corresponding ester. It is a classical approach, well documented in literature, that can guarantee good yields with many ketones and aldehydes. The reagent is also used to generate acylsilanes with electrophiles, such as epoxides.
Synonyms: silane, 1,3-dithian-2-yltrimethyl- (9CI); silane, m-dithian-2-yltrimethyl- (8CI); (1,3-dithian-2-yl)trimethylsilane
Selected publication
-
2-Trimethylsilyl-1,3-Dithiane.
Benbow J.; Foley M.; Smith A. Encyclopedia of Reagents for Organic Synthesis 2007. DOI: 10.1002/047084289X.rt299.pub2