CAS 339166-89-1, Cat. No EN300-12596901
Reagent for radical annulation
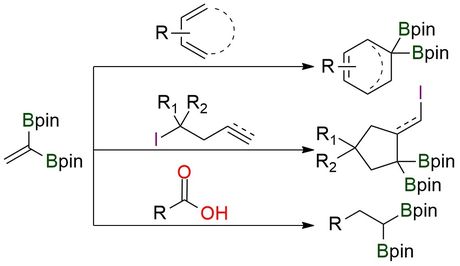
2,2-Bis(pinacolboryl)ethylene is a gem-diborylalkene used in stereoselective Diels–Alder reactions and radical annulations1,2. It enables the synthesis of 1,1-bisborylcyclohexenes and γ-iodo-gem-diborylated cyclopentanes, serving as key intermediates in organoboron chemistry3. The reagent is also valuable in ring-opening metathesis polymerization (ROMP), producing polyboron-based materials with unique properties. Its applications extend to bioactive molecule synthesis and polymer science, making it an essential tool in modern synthetic chemistry. The reagent is air-stable, non-toxic, and easy to handle, ensuring broad usability.
Synonyms: 2,2'-ethenylidenebis[4,4,5,5-tetramethyl-1,3,2-dioxaborolane]; 4,4,5,5-tetramethyl-2-[1-(tetramethyl-1,3,2-dioxaborolan-2-yl)ethenyl]-1,3,2-dioxaborolane; 1,3,2-dioxaborolane, 2,2'-ethenylidenebis[4,4,5,5-tetramethyl-; 4,4,5,5-tetramethyl-2-(1-(tetramethyl-1,3,2-dioxaborolan-2-yl)ethenyl)-1,3,2-dioxaborolane; 2,2'-(ethene-1,1-diyl)bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)
Selected publications
-
Stereoselective Diels–Alder Reactions of Gem-Diborylalkenes: Toward the Synthesis of Gem- Diboron-Based Polymers.
Eghbarieh N.; Hanania N.; Zamir A.; Nassir M.; Stein T.; Masarwa A. J Am Chem Soc 2021, 143 (16), 6211–6220. DOI: 10.1021/jacs.1c01471
-
Photoredox‐Mediated Reaction of Gem ‐Diborylalkenes: Reactivity Toward Diverse 1,1‐Bisborylalkanes.
Kumar N.; Eghbarieh N.; Stein T.; Shames A. I.; Masarwa A. Chemistry – A European Journal 2020, 26 (24), 5360–5364. DOI: 10.1002/chem.202000603
-
Access to Γ‐Iodo‐Gem‐Diborylated Cyclopentanes and to Bicyclic Cyclopropanes.
Circule D.; Dénès F.; Renaud P. Adv Synth Catal 2024, 366 (13), 2945–2955. DOI: 10.1002/adsc.202400340