CAS 1214264-88-6, Cat. No EN300-7424113
Reagent for borylation reactions
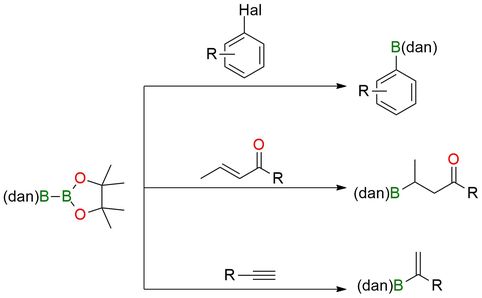
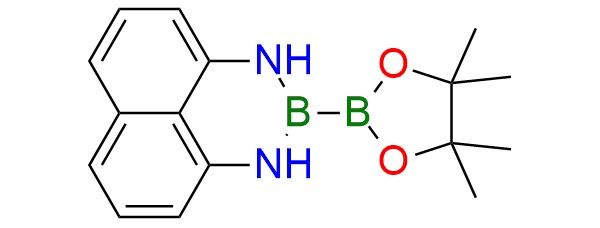
1-Pinacolato-2-(1,8)diamo-naphthalenylborane ((pin)B−B(dan)) is a stable diazaborine–boronic ester reagent, which is used in selective diborylation reactions of alkenes, alkynes, diazoalkanes. It also undergoes Suzuki-Miyaura coupling to give B(dan) derivatives1,2. The reagent tolerates aryl and heteroaryl bromides, iodides, and activated chlorides, including electron-rich, electron-poor, and sterically hindered partners (alkenes, alkynes, diazoalkanes). Compatible with esters, nitriles, amides, and heterocycles, it shows high chemoselectivity for boronic ester coupling without affecting the diazaborine unit.
Synonyms: (pin)B−B(dan); 3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2,4-diaza-3-boratricyclo[7.3.1.05,13]trideca-1(12),5,7,9(13),10-pentaene; 3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2,4-diaza-3-boratricyclo[7.3.1.0(1)(3)]trideca-1(13),5,7,9,11-pentaene ; (dan)B-B(pin)
Selected publications
-
Copper-Catalyzed B(Dan)-Installing Allylic Borylation of Allylic Phosphates.
Yoshida, H.; Murashige, Y.; Osaka, I. Adv Synth Catal 2019, 361 (10), 2286–2290. DOI: 10.1002/adsc.201900342
-
Direct Introduction of a Naphthalene-1,8-Diamino Boryl [B(Dan)] Group by a Pd-Catalysed Selective Boryl Transfer Reaction.
Xu, L.; Li, P. Chemical Communications 2015, 51 (26), 5656–5659. DOI: 10.1039/c5cc00231a