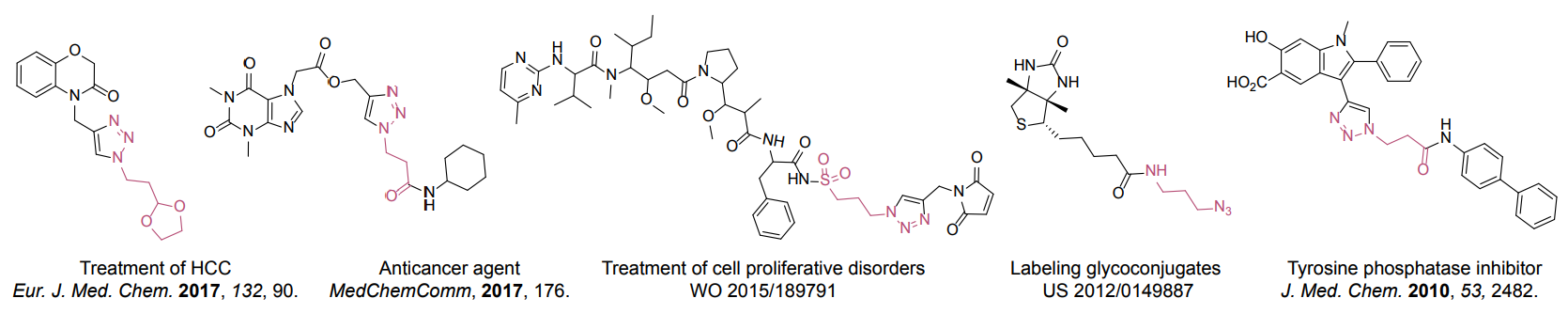
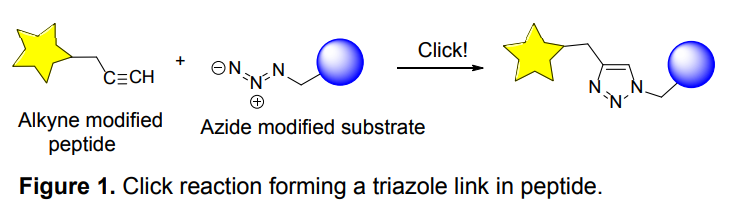
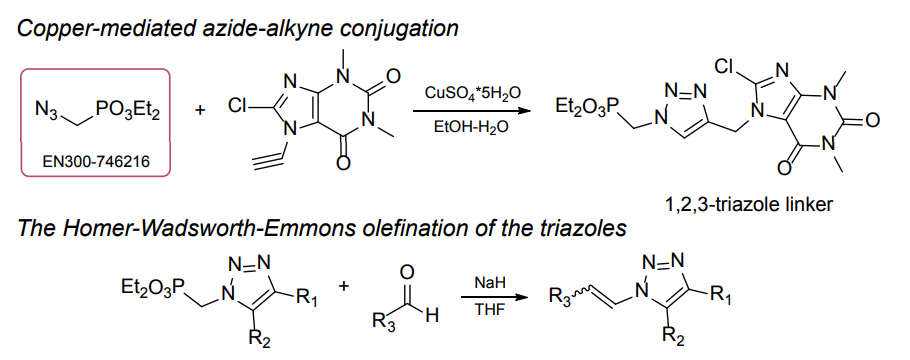
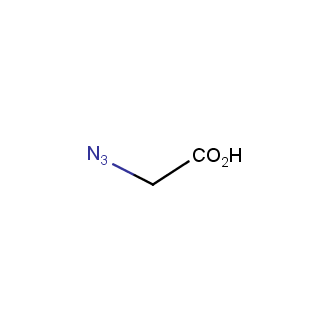
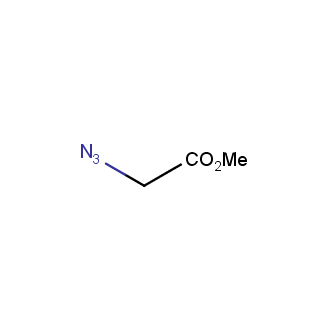
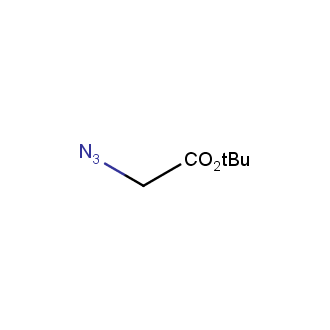
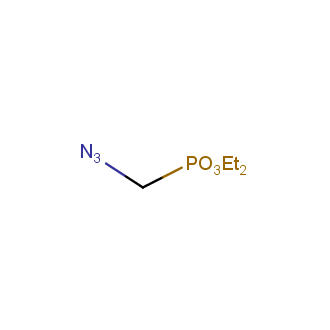
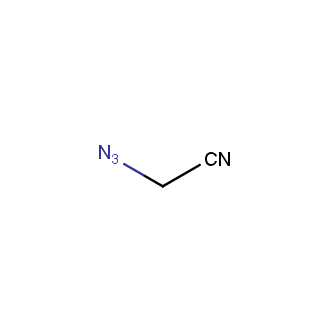
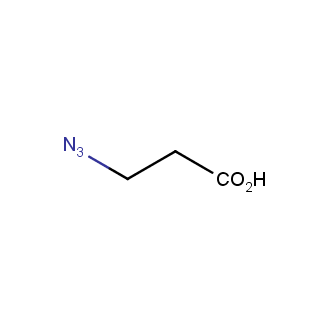
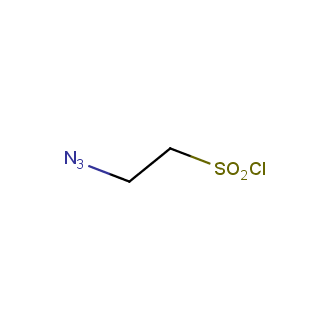
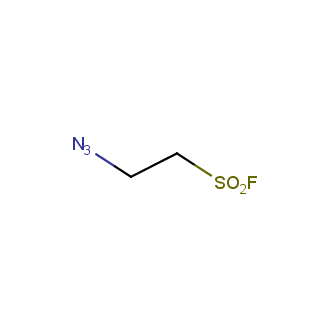
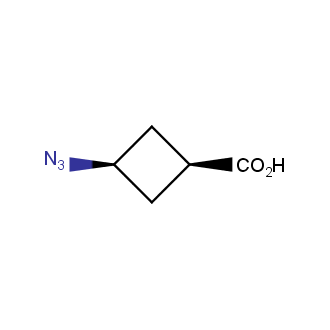
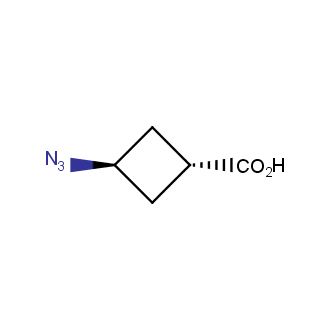
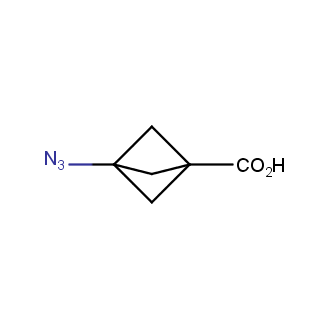
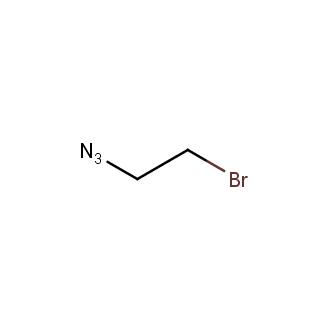
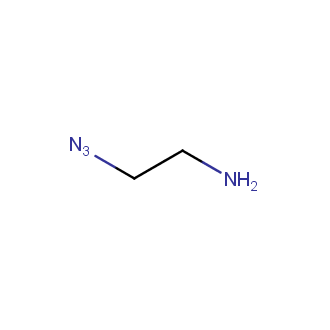
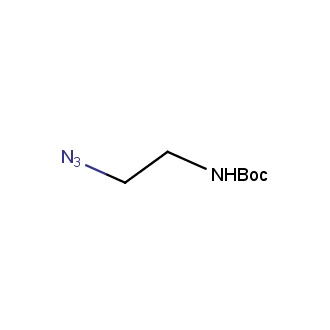
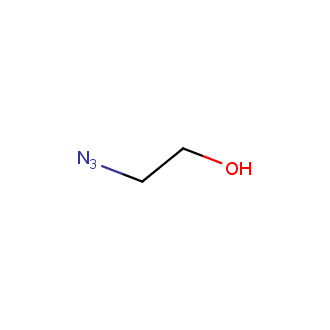
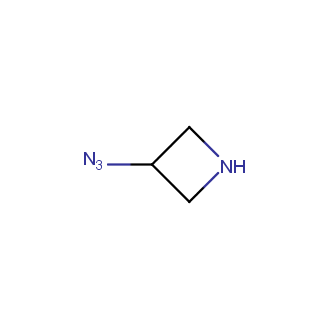
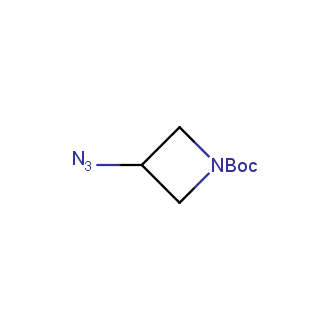
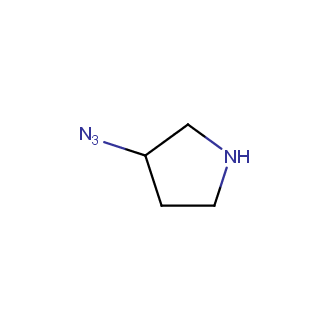
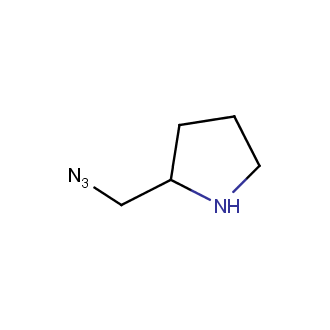
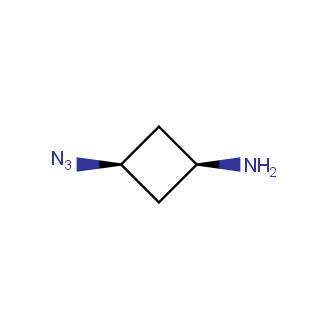
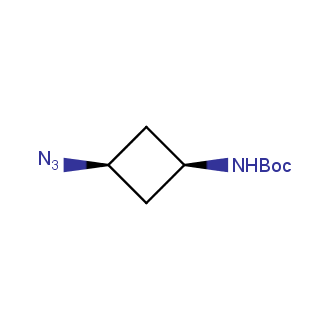
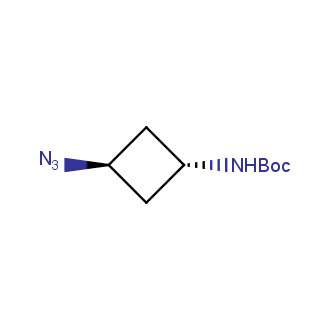
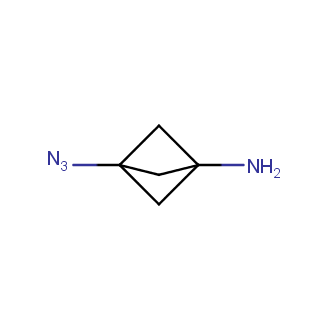
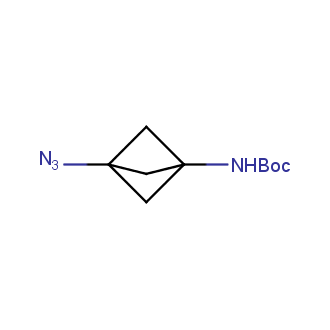
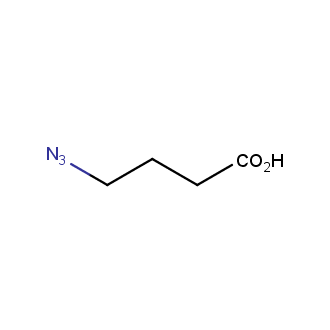
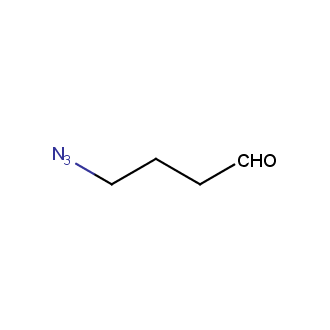
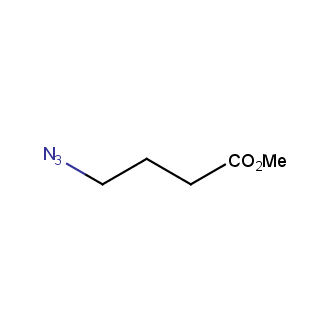
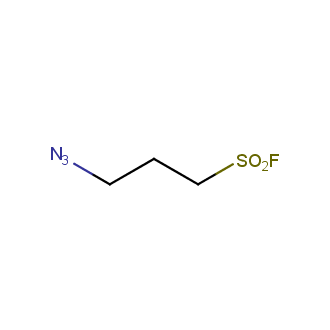
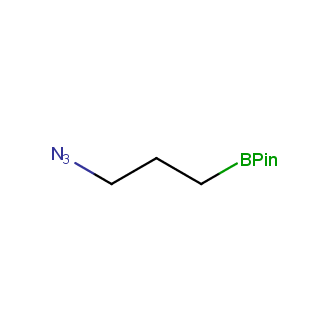
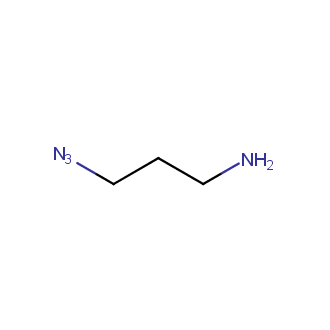
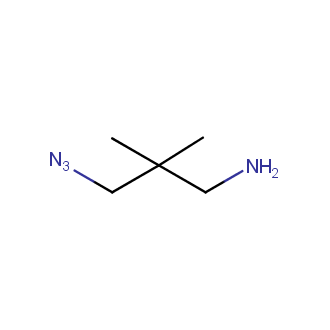
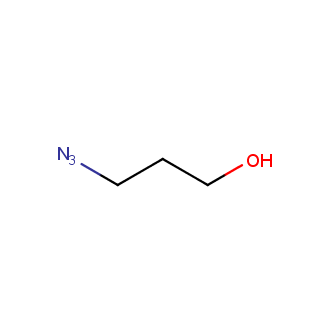
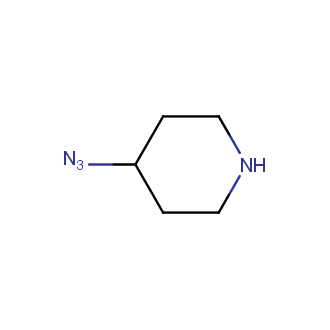
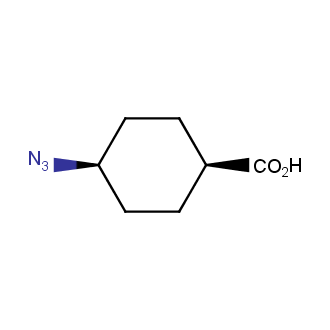
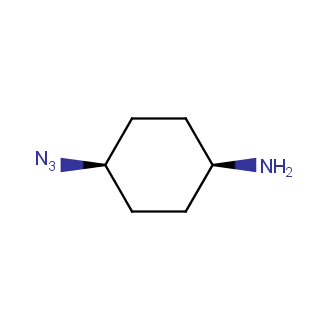
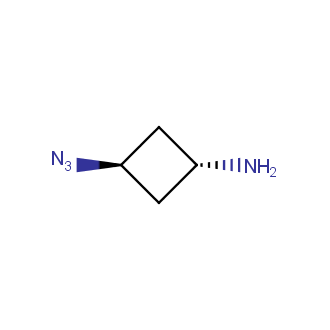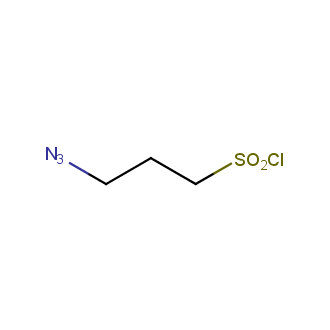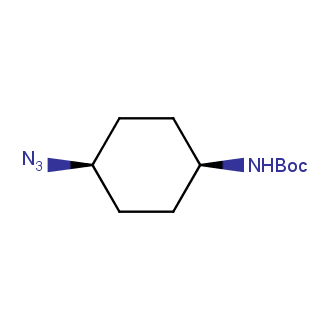Organic azides became enormously popular for their participation in the Cu(I)-catalyzed Huisgen azide-alkyne 1,3R09;dipolar cycloaddition reaction – “click chemistry”. 1,2,3-triazole function formed by click reaction between an azide and alkyne bears a physicochemical resemblance to the amide bond. Besides, “click chemistry” involves functionalities that can be introduced in small molecules and into specific locations in biomolecules. “Click chemistry” continues to gain popularity and is used in a variety of research fields with significant contributions to the fields of bioconjugation and drug discovery.
Advantages
- Wide in scope.
- Form stable products.
- Give very high yields.
- The presence of the azide-group and a functional group allows the molecule to be modified before or after the click reaction.
Our offer
>100 different building blocks in multi gram amounts in stock. We also have designed a library azide-containing building blocks for drug discovery programs. These molecules can be synthesized upon request within 4-6 weeks.