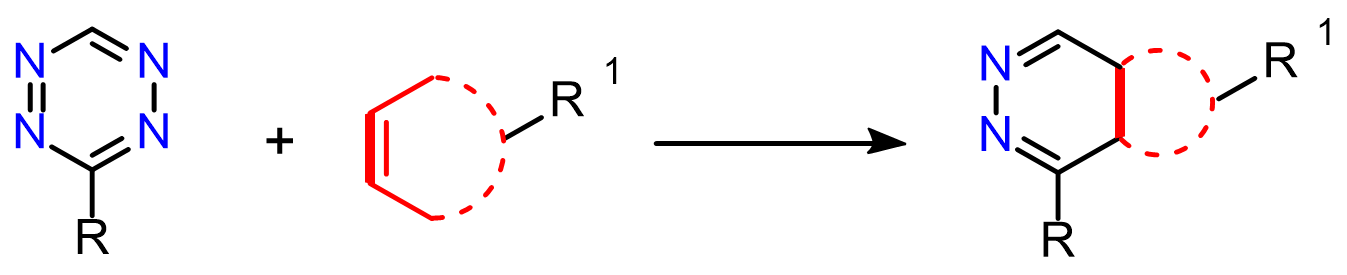
A well-known bioorthogonal reaction is the Inverse Electron Demand Diels–Alder (IEDDA) cycloaddition between strained alkenes (such as norbornenes, trans-cyclooctenes, and cyclopropenes) and 1,2,4,5-tetrazines (electron-deficient dienes). This reaction is exceptionally fast, does not require a catalyst, and proceeds cleanly in complex biological systems without perturbing the biological milieu.1
The two major applications of strained alkene–tetrazine cycloadditions include: bioconjugation2 (for example, linking small molecules to antibodies or proteins) and click-to-release chemistry (enabling the controlled release of a functional payload upon reaction between tetrazine and trans-cyclooctene).3
Download SD files
Enamine offers a range of functionalized strained alkenes, including cyclopropenes and trans-cyclooctenes, available from stock. We also provide custom synthesis of strained alkenes designed to meet your specific needs.
-
Inverse electron demand Diels–Alder (iEDDA)-initiated conjugation: a (high) potential click chemistry scheme
A.-C. Knall, C. Slugovc, Chem. Soc. Rev. 2013, 42, 5131. DOI: 10.1039/C3CS60049A -
Biomedical applications of copper-free click chemistry: in vitro, in vivo, and ex vivo.
Kim E., Koo H. Chem. Sci. 2019, 10, 7835. DOI: 10.1039/C7CS00184C -
Click to release: instantaneous doxorubicin elimination upon tetrazine ligation
R. M. Versteegen, R. Rossin, W. ten Hoeve, H. M. Janssen, M. S. Robillard Angew. Chem. Int. Ed. 2013,52, 14112–14116. DOI: 10.1002/anie.201305969
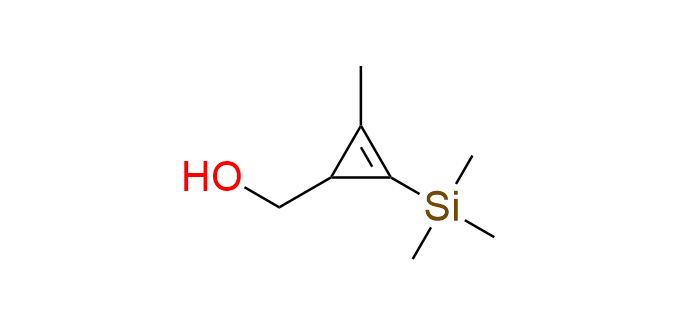
[2-methyl-3-(trimethylsilyl)cycloprop-2-en-1-yl]methanol
CAS 29916-92-5, Cat. No EN300-295436
SiMe₃-protected cyclopropenyl methanol serves as a valuable building block for incorporating a cyclopropene moiety into target molecules. Derivatives of this compound exhibit high reaction rates in inverse electron-demand Diels–Alder (IEDDA) reactions with tetrazines. The presence of a methyl group is crucial for maintaining the stability of the cyclopropene unit. The SiMe₃ group functions as a protective group during the synthesis of derivatives and can be readily removed under mild conditions. The hydroxyl group is used as an attachment point through simple chemical transformations.
- A M. Patterson, L. A. Nazarova, B. Xie, D. N. Kamber and J. A. Prescher, J. Am. Chem. Soc., 2012, 134, 18638–18643. DOI: 10.1021/ja3060436
- Yang, J. Šečkutė, C. M. Cole and N. K. Devaraj, Angew. Chem., Int. Ed., 2012, 51, 7476–7479. DOI: 10.1002/anie.201202122
- Yang, Y. Liang, J. Šečkutė, K. N. Houk and N. K. Devaraj, Chem. Eur. J., 2014, 20, 3365–3375. DOI: 10.1002/chem.201304225
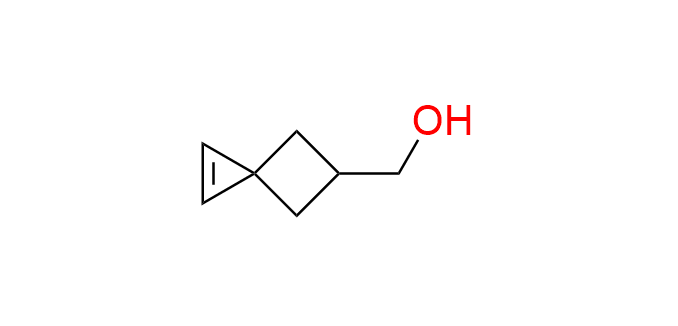
{spiro[2.3]hex-1-en-5-yl}methanol
CAS 1621611-39-9, Cat. No EN300-7604414
This strained spirocyclic cyclopropene exhibits enhanced reactivity compared to simpler monocyclic derivatives, both in the IEDDA reaction with tetrazines and as a dipolarophile in the photoinduced cycloaddition with tetrazoles. Its small size and increased water solubility make it an attractive building block for the development of cyclopropene-based probes. The hydroxyl group of the compound is used as an attachment point through simple chemical transformations.
- An, H.-Y.; Wu, T. M.; Lewandowski and Q. Lin, Chem. Commun., 2018, 54, 14005–14008. DOI: 10.1039/C8CC07432A
- Yu and Q. Lin, J. Am. Chem. Soc., 2014, 136, 4153–4156. DOI: 10.1021/ja5012542
- Son, D.; Kim, S.; Kim, W. G.; Byun and S. B. Park, Angew. Chem., Int. Ed., 2025, 64, e202421982. DOI: 10.1002/anie.202421982
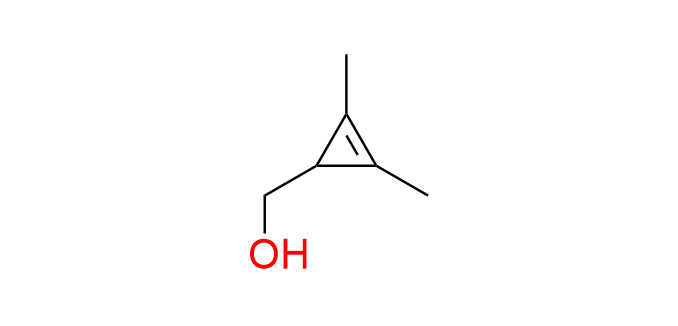
(2,3-dimethylcycloprop-2-en-1-yl)methanol
CAS 33283-69-1, Cat. No EN300-7585587
Dimethyl cyclopropene derivatives exhibit enhanced stability both during chemical transformations and in biological environments. Another advantage is the small size of the fragment. On the other hand, they show low to moderate reactivity in the IEDDA reaction with tetrazines. In other words, the balance between stability and reactivity is tipped toward stability. The hydroxyl group of the compound is used as an attachment point through simple chemical transformations.
- Baalmann, L. Neises, S. Bitsch, H. Schneider, L. Deweid, P. Werther, N. Ilkenhans, M. Wolfring, M. J. Ziegler, J. Wilhelm, H. Kolmar and R. Wombacher, Angew. Chem., Int. Ed., 2020, 59, 12885–12893. DOI: 10.1002/anie.201915079
- M. Patterson, L. A. Nazarova, B. Xie, D. N. Kamber and J. A. Prescher, J. Am. Chem. Soc., 2012, 134, 18638–18643. DOI: 10.1021/ja3060436
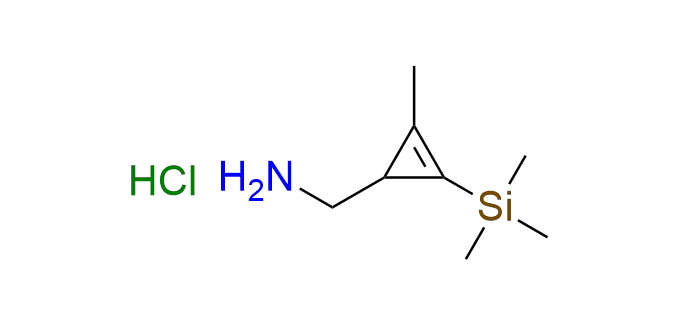
1-[2-methyl-3-(trimethylsilyl)cycloprop-2-en-1-yl]methanamine hydrochloride
CAS 2172212-79-0 (hydrochloride), 1466419-88-4 (base), Cat. No EN300-1662341
SiMe₃-protected aminomethyl cyclopropene serves as a valuable building block for incorporating a cyclopropene moiety into target molecules. Derivatives of this compound exhibit high reaction rates in inverse electron-demand Diels–Alder (IEDDA) reactions with tetrazines, along with remarkable stability. The presence of a methyl group is crucial for maintaining the stability of the cyclopropene unit. The SiMe₃ group acts as a protective group during the synthesis of derivatives and can be readily removed under mild conditions. The amino group of the compound serves as an attachment point through simple chemical transformations.
- Yang, Y. Liang, J. Šečkutė, K. N. Houk and N. K. Devaraj, Chem. Eur. J., 2014, 20, 3365–3375. DOI: 10.1002/chem.201304225
- Seul, D. Lamade, P. Stoychev, M. Mijic, R. T. Michenfelder, L. Rieger, P. Geng and H.-A. Wagenknecht, Angew. Chem., Int. Ed., 2024, 63, e202403044. DOI: 10.1002/anie.202403044
- Simon, C. Lion, C. Spriet, F. Baldacci-Cresp, S. Hawkins, C. Biot, Angew. Chem. Int. Ed., 2018, 57, 16665. DOI: 10.1002/anie.201808493
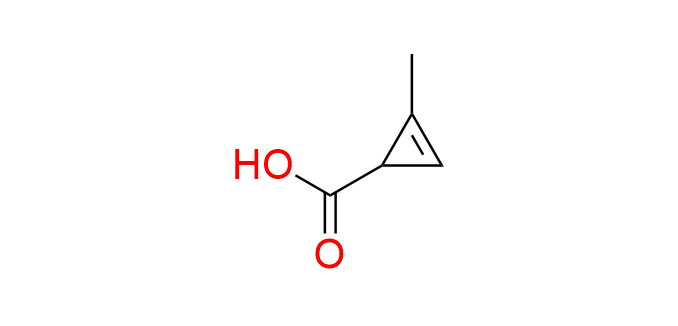
2-methylcycloprop-2-ene-1-carboxylic acid
CAS 39492-17-6, Cat. No EN300-118360
A simple cyclopropene carboxylic acid exhibits sluggish reactivity in the IEDDA reaction with tetrazines and o-quinones. Nonetheless, its compact molecular size remains a significant advantage. Incorporation of a methyl group is crucial for achieving an optimal balance between the reactivity and stability of the cyclopropene moiety. The carboxyl group of the compound serves as an attachment point through simple chemical transformations.
- M. Patterson, L. A. Nazarova, B. Xie, D. N. Kamber and J. A. Prescher, J. Am. Chem. Soc., 2012, 134, 18638–18643. DOI: 10.1021/ja3060436
- Yang, J. Šečkutė, C. M. Cole and N. K. Devaraj, Angew. Chem., Int. Ed., 2012, 51, 7476–7479. DOI: 10.1002/anie.201202122
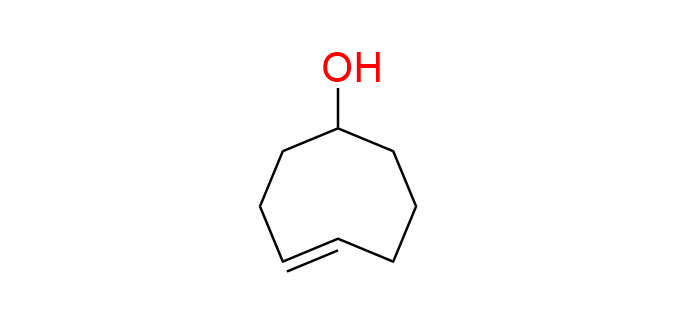
(4E)-cyclooct-4-en-1-ol (TCO-OH; (4E)-4-Cycloocten-1-ol; trans-Cyclooctenol)
CAS 85081-69-2, Cat. No EN300-1609058
The inverse electron-demand Diels–Alder (IEDDA) reaction between trans-cyclooctenes and tetrazines is the fastest known bioorthogonal reaction. 4-TCO-OH is a well-known, highly reactive yet simple dienophile commonly used for bioconjugation.
- Devaraj, S. Hilderbrand, R. Upadhyay, R. Mazitschek and R. Weissleder, Angew. Chem., Int. Ed., 2010, 49, 2869–2872. DOI: 10.1002/anie.200906120
- Baalmann, L. Neises, S. Bitsch, H. Schneider, L. Deweid, P. Werther, N. Ilkenhans, M. Wolfring, M. J. Ziegler, J. Wilhelm, H. Kolmar and R. Wombacher, Angew. Chem., Int. Ed., 2020, 59, 12885–12893. DOI: 10.1002/anie.201915079
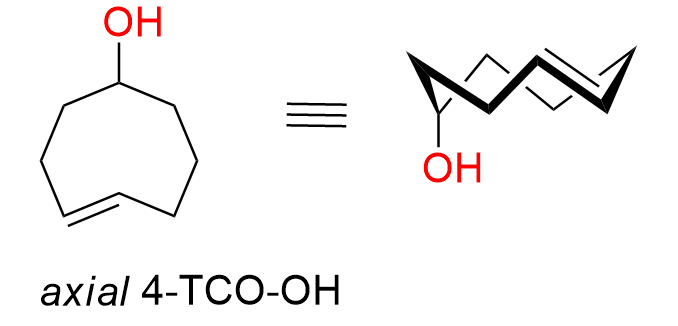
Axial-(4E)-cyclooct-4-en-1-ol
CAS: 1018976-14-1 (axial isomer), 85081-69-2 (axial+equatorial isomers), Cat No EN300-21978676
Synonyms: Axial-(4E)-cyclooct-4-en-1-ol; ax-TCO-OH; axial TCO-OH; TCO-OH (axial); axial-(E)-cyclooct-4-enol; rel-(1R,4E,pS)-Cyclooct-4-enol
4-TCO-OH is a well-known, highly reactive yet simple dienophile commonly used for bioconjugation. The axial diastereomer is more reactive in the IEDDA reaction with tetrazines. This compound is commonly used for bioconjugation.
- Rossin, S. M. van den Bosch, W. ten Hoeve, M. Carvelli, R. M. Versteegen, J. Lub and M. S. Robillard, Bioconjugate Chem., 2013, 24, 1210–1217. DOI: 10.1021/bc400153y
- Baalmann, L. Neises, S. Bitsch, H. Schneider, L. Deweid, P. Werther, N. Ilkenhans, M. Wolfring, M. J. Ziegler, J. Wilhelm, H. Kolmar and R. Wombacher, Angew. Chem., Int. Ed., 2020, 59, 12885–12893. DOI: 10.1002/anie.201915079
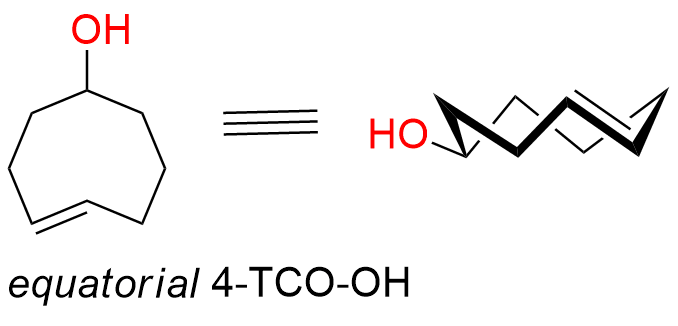
equatorial-(4E)-cyclooct-4-en-1-ol
CAS: 1018976-12-9 (equatorial diastereomer), 85081-69-2 (axial+equatorial isomers), Cat No EN300-25977034
Synonyms: eq-TCO-OH; TCO-OH (equatorial); equatorial-(E)-cyclooct-4-enol; rel-(1R,4E,pR)-Cyclooct-4-enol
4-TCO-OH is a well-known, highly reactive yet simple dienophile commonly used for bioconjugation. The equatorial diastereomer is less reactive in the IEDDA reaction with tetrazines. Despite this, the compound is still widely used for bioconjugation applications.
- Rossin, S. M. van den Bosch, W. ten Hoeve, M. Carvelli, R. M. Versteegen, J. Lub and M. S. Robillard, Bioconjugate Chem., 2013, 24, 1210–1217. DOI: 10.1021/bc400153y
- Baalmann, L. Neises, S. Bitsch, H. Schneider, L. Deweid, P. Werther, N. Ilkenhans, M. Wolfring, M. J. Ziegler, J. Wilhelm, H. Kolmar and R. Wombacher, Angew. Chem., Int. Ed.,2020, 59, 12885–12893. DOI: 10.1002/anie.201915079
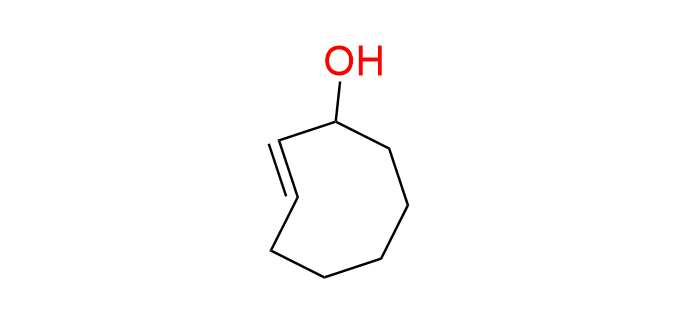
(2E)-cyclooct-2-en-1-ol
CAS: 1414375-00-0, Cat No EN300-45434666
Synonyms: (E)-cyclooct-2-enol; (E)-cyclooct-2-en-1-ol; trans-cyclooct-2-enol; r-TCO; release-TCO;
Trans-cyclooctene, bearing a leaving group in the allylic position, is well known for its ability to eliminate this group following an IEDDA reaction with tetrazines. This property forms the foundation of the click-to-release methodology—a rapidly advancing strategy for prodrug delivery.
- M. Versteegen, R. Rossin, W. ten Hoeve, H. M. Janssen and M. S. Robillard, Angew. Chem., Int. Ed., 2013, 52, 14112–14116. DOI: 10.1002/anie.201305969
- M. Versteegen, W. ten Hoeve, R. Rossin, M. A. R. de Geus, H. M. Janssen and M. S. Robillard, Angew. Chem., Int. Ed.,2018, 57, 10494. DOI: 10.1002/anie.201800402
- J. C. Sarris, T. Hansen, M. A. R. de Geus, E. Maurits, W. Doelman, H. S. Overkleeft, J. D. C. Codée, D. V. Filippov, S. I. van Kasteren, Chem. Eur. J. 2018, 24, 18075. DOI: 10.1002/chem.201803839
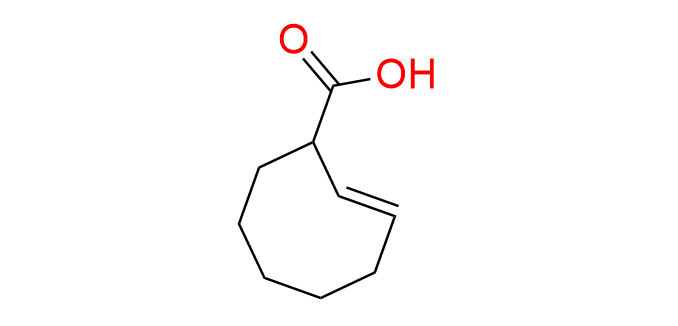
(2E)-cyclooct-2-ene-1-carboxylic acid
CAS: 1414375-01-1, Cat No EN300-47214031
A trans-cyclooctene-based carboxylic acid is employed in the click-to-release methodology. A target compound bearing a hydroxyl group can be caged via esterification with this acid and subsequently uncaged following an IEDDA reaction with tetrazines.
- Wu, K. Wu, F. Gaye and M. Royzen, Org. Lett., 2020, 22, 6041–6044. DOI: 10.1021/acs.orglett.0c02129
Can't find the substance you need? Contact us for custom synthesis solutions!