Among the most well-known bioorthogonal transformations is the Strain-Promoted Azide–Alkyne Cycloaddition (SPAAC).
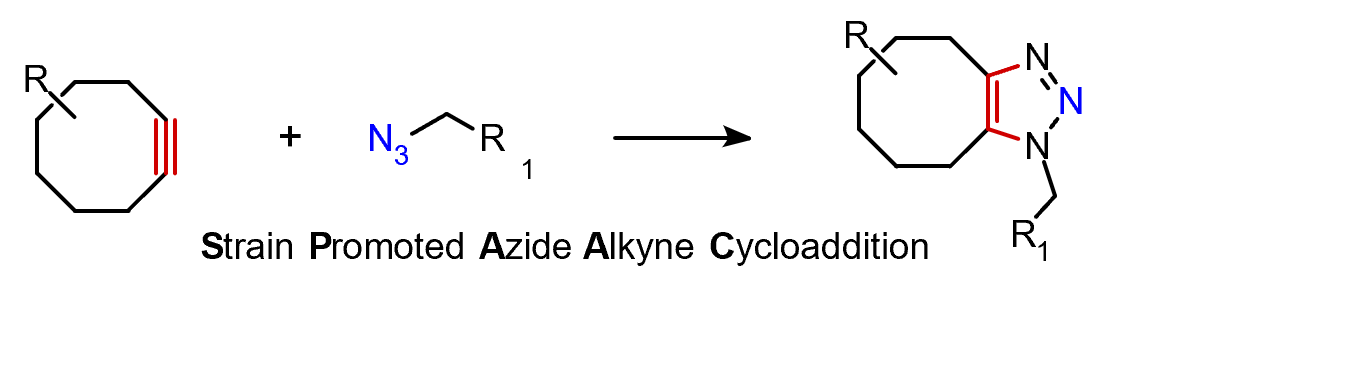
One component of the reaction, alkyl azide, is a common class of compounds that are commercially available or can be synthesized through simple chemical transformations. On the other hand, alkynes need to be strained to react at physiological temperatures without the use of a catalyst. Cyclooctynes and hetero-cycloalkynes are the smallest cyclic alkynes stable enough for practical applications. Yet, their inherent ring strain is what really sets them apart, enabling [3+2] cycloadditions with azides and other dipoles (like nitrile oxides, nitrile imines, and nitrones) without the need for catalysis, even at biological temperatures.1
Enamine offers a diverse selection of cyclooctynes, all readily available from stock, to support your research.
Download SD files
We also provide custom synthesis of cyclooctynes, designed to meet your specific needs.
Below are some of the most relevant cyclootyne-based building blocks for your research. Each is available from stock.
-
Biomedical applications of copper-free click chemistry: in vitro, in vivo, and ex vivo.
Kim E. and Koo H. Chem. Sci. 2019, 10, 7835. DOI: 10.1039/C9SC03368H
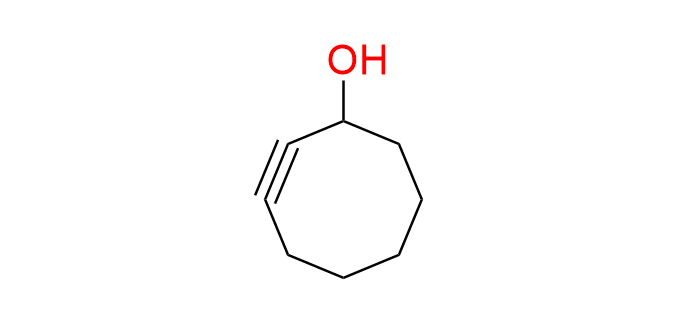
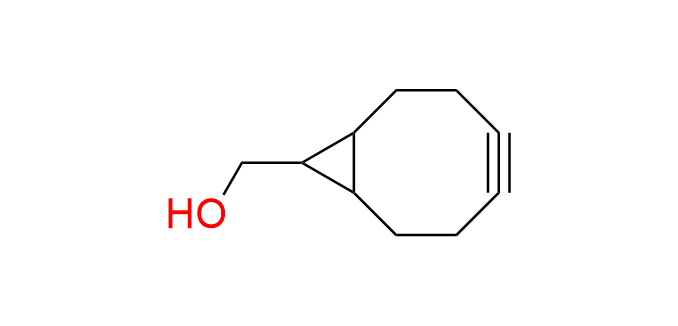
cyclooct-2-yn-1-ol
CAS 29916-92-5, Cat. No EN300-7551522
The simplest and smallest 1st-generation cyclooctyne derivative.
- T. Hagendorn and S. Bräse, Eur. J. Org. Chem., 2014, 1280. DOI: 10.1002/ejoc.201301375
- D. Kang and J. Kim, J. Am. Chem. Soc., 2021, 143, 5616. DOI: 10.1021/jacs.1c00885
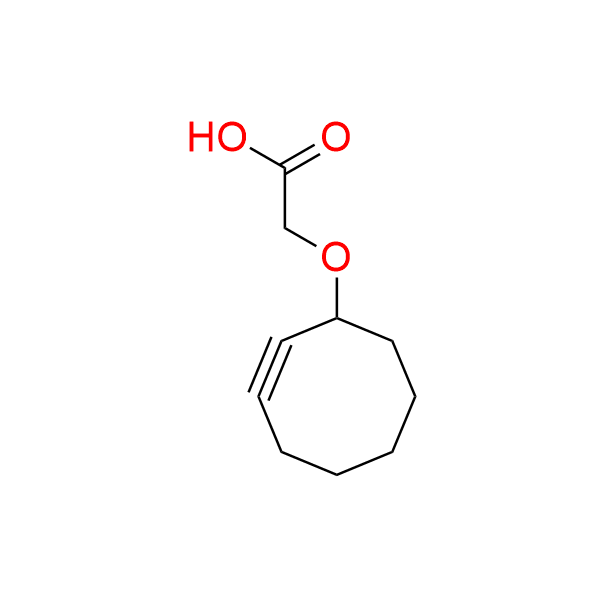
2-(cyclooct-2-yn-1-yloxy)acetic acid
CAS 917756-42-4, Cat. No EN300-179238
This compound is one of the first cyclooctynes used for bioorthogonal ligation with azides. It has a small size and a simple structure. These features make it versatile for various bioorthogonal transformations.
- H. L. Evans, R. L. Slade, L. Carroll, G. Smith, Q.-D. Nguyen, L. Iddon, N. Kamaly, H. Stöckmann, F. J. Leeper, E. O. Aboagye and A. C. Spivey, Chem. Commun., 2012, 48, 991. DOI: 10.1039/C1CC16220A
- M. Burk, S. Rothstein and P. Dubé, Org. Process Res. Dev., 2018, 22, 108. DOI: 10.1021/acs.oprd.7b00275
- N. J. Agard, J. M. Baskin, J. A. Prescher, A. Lo and C. R. Bertozzi, ACS Chem. Biol., 2006, 1, 644. DOI: 10.1021/cb6003228
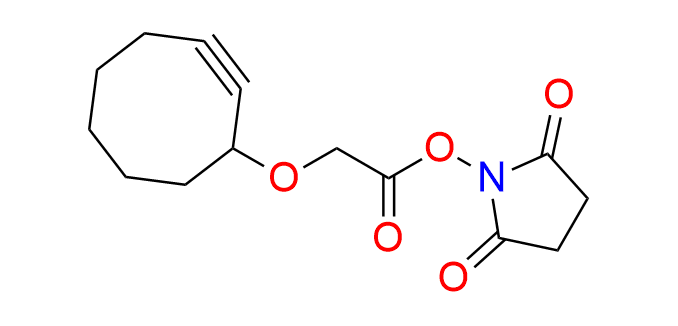
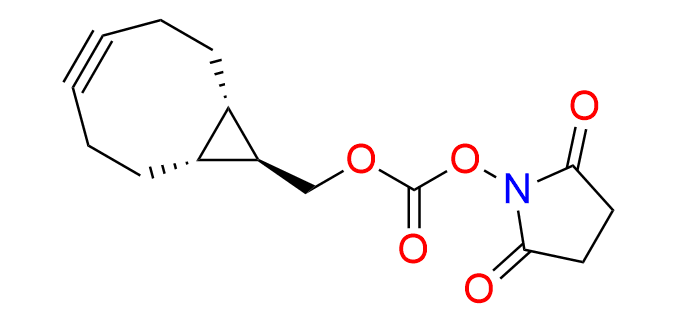
2,5-dioxopyrrolidin-1-yl 2-(cyclooct-2-yn-1-yloxy)acetate
CAS 1425803-45-7, Cat. No EN300-19594192
This compound is an activated ester of cyclooctynyloxyacetic acid, EN300-179238. It can be used directly for the acylation of amines or alcohols, facilitating the construction of molecules of interest for bioorthogonal ligation.
- C.-H. Lai, T.-C. Chang, Y.-J. Chuang, D.-L. Tzou and C.-C. Lin, Bioconjugate Chem., 2013, 24, 1698. DOI: 10.1021/bc400219t
- M. Burk, S. Rothstein and P. Dubé, Org. Process Res. Dev., 2018, 22, 108. DOI: 10.1021/acs.oprd.7b00275
- T. Wang, J. G. Vineberg, T. Honda and I. Ojima, Bioorg. Chem., 2018, 76, 458. DOI: 10.1016/j.bioorg.2017.12.018
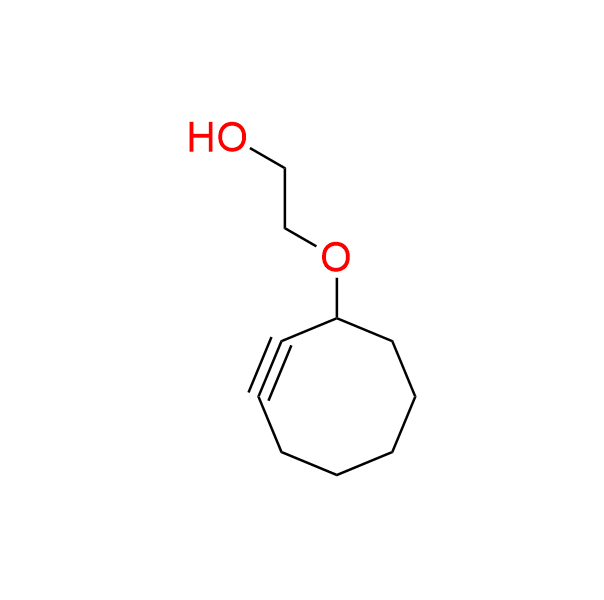
2-(cyclooct-2-yn-1-yloxy)ethan-1-ol
CAS 1309581-54-1, Cat. No EN300-5256850
A small 1st-generation cyclooctyne with an alcohol moiety.
- T. Plass, S. Milles, C. Koehler, C. Schultz and E. A. Lemke, Angew. Chem., Int. Ed., 2011, 50, 3878. DOI: 10.1002/anie.201008178
- D. Guan, Y. Kurra, W. Liu and Z. Chen, Chem. Commun., 2015, 51, 2522. DOI: 10.1039/C4CC09179E
BCN-OH
CAS 1379662-52-8, Cat. No EN300-178372
Synonyms: [bicyclo[6.1.0]non-4-yn-9-yl]methanol
BCN-OH is the most famous cyclooctyne, known for its balance between high reactivity and small size.
The first and most studied transformation involving BCN-OH is the Strain-Promoted Azide–Alkyne Cycloaddition (SPAAC).
Nevertheless, it also finds application in cycloadditions with tetrazines and nitrile imines.
- D. Kim, H. Son and S. B. Park, Angew. Chem., Int. Ed., 2023, 62, e202310665. DOI: 10.1002/anie.202310665
- M. Fang, G. S. Kumar, S. Racioppi, H. Zhang, J. D. Rabb, E. Zurek and Q. Lin, J. Am. Chem. Soc., 2023, 145, 9959. DOI: 10.1021/jacs.2c12325
- G. S. Kumar, S. Racioppi, E. Zurek and Q. Lin, J. Am. Chem. Soc., 2022, 144, 57. DOI: 10.1021/jacs.1c10354
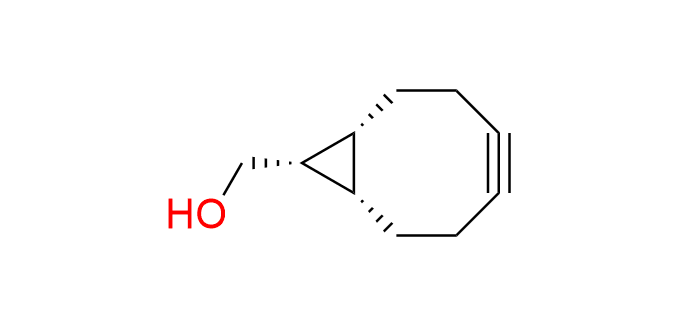
endo-BCN-OH
CAS 1263166-90-0, Cat. No EN300-342715
Synonyms: [(1R,8S,9S)-bicyclo[6.1.0]non-4-yn-9-yl]methanol; BCN-OH; (1α,8α,9β)-Bicyclo[6.1.0]non-4-yne-9-methanol; endo-Bicyclo[6.1.0]non-4-yn-9-ylmethanol; (1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethanol
BCN-OH is the most famous cyclooctyne, known for its balance between high reactivity and small size. The first and most studied transformation involving BCN-OH is the Strain-Promoted Azide–Alkyne Cycloaddition (SPAAC). It also finds application in cycloadditions with tetrazines and nitrile imines. Endo-BCN-OH is the most reactive isomer in SPAAC.
- J. Dommerholt, S. Schmidt, R. Temming, L. J. A. Hendriks, F. P. J. T. Rutjes, J. C. M. van Hest, D. J. Lefeber, P. Friedl and F. L. van Delft, Angew. Chem., Int. Ed., 2010, 49, 9422. DOI: 10.1002/anie.201003761
- M. F. Debets, S. S. van Berkel, J. Dommerholt, A. J. Dirks, F. P. J. T. Rutjes and F. L. van Delft, Acc. Chem. Res., 2011, 44, 805. DOI: 10.1021/ar200059z
- M. Baalmann, L. Neises, S. Bitsch, H. Schneider, L. Deweid, P. Werther, N. Ilkenhans, M. Wolfring, M. J. Ziegler, J. Wilhelm, H. Kolmar and R. Wombacher, Angew. Chem., Int. Ed., 2020, 59, 12885. DOI: 10.1002/anie.201915079
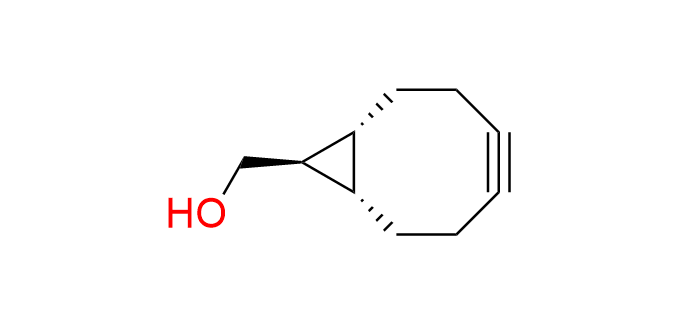
exo-BCN-OH
CAS 1263291-41-3, Cat. No EN300-378398
Synonyms: [(1R,8S,9R)-bicyclo[6.1.0]non-4-yn-9-yl]methanol; (1α,8α,9α)-Bicyclo[6.1.0]non-4-yne-9-methanol; exo-Bicyclo[6.1.0]non-4-yn-9-ylmethanol
BCN-OH is the most famous cyclooctyne, known for its balance between high reactivity and small size. The first and most studied transformation involving BCN-OH is the Strain-Promoted Azide–Alkyne Cycloaddition (SPAAC). It also finds application in cycloadditions with tetrazines and nitrile imines.
- J. Dommerholt, S. Schmidt, R. Temming, L. J. A. Hendriks, F. P. J. T. Rutjes, J. C. M. van Hest, D. J. Lefeber, P. Friedl and F. L. van Delft, Angew. Chem., Int. Ed., 2010, 49, 9422. DOI: 10.1002/anie.201003761
- X. Li, Z. Liu and S. Dong, RSC Adv., 2017, 7, 44470. DOI: 10.1039/C7RA08136G
- K. Lang, L. Davis, S. Wallace, M. Mahesh, D. J. Cox, M. L. Blackman, J. M. Fox and J. W. Chin, J. Am. Chem. Soc., 2012, 134, 10317. DOI: 10.1021/ja302832g
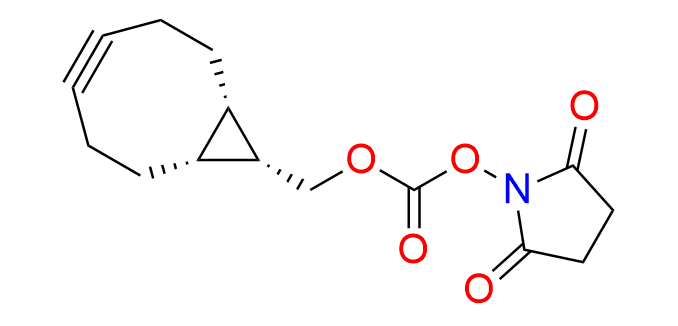
endo-BCN-NHS carbonate
CAS 1426827-79-3, Cat. No EN300-18974406
Synonyms: BCN-NHS; [(1R,8S,9S)-bicyclo[6.1.0]non-4-yn-9-yl]methyl 2,5-dioxopyrrolidin-1-yl carbonate; (1α,8α,9β)-Bicyclo[6.1.0]non-4-yn-9-ylmethyl 2,5-dioxo-1-pyrrolidinyl carbonate; rel-(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyl (2,5-dioxopyrrolidin-1-yl) carbonate
Endo-BCN-NHS carbonate is a ready-to-use derivative of the well-known cyclooctyne, BCN-OH, EN300-342715, which is recognized for its balance of high reactivity and compact size.
The primary and most extensively studied reaction involving BCN derivatives is the Strain-Promoted Azide–Alkyne Cycloaddition (SPAAC).
Endo-BCN derivatives are more reactive isomers in SPAAC, comparable to exo-BCN. They are also utilized in cycloadditions with tetrazines, tetrazoles and nitrile imines.
- A. Borrmann, S. Milles, T. Plass, J. Dommerholt, J. M. M. Verkade, M. Wießler, C. Schultz, J. C. M. van Hest, F. L. van Delft and E. A. Lemke, ChemBioChem, 2012, 13, 2094. DOI: 10.1002/cbic.201200407
- T. Komatsu, E. Kyo, H. Ishii, K. Tsuchikama, A. Yamaguchi, T. Ueno, K. Hanaoka and Y. Urano, J. Am. Chem. Soc., 2014, 142, 15644. DOI: 10.1021/jacs.0c05331
- G. S. Kumar, S. Racioppi, E. Zurek and Q. Lin, J. Am. Chem. Soc., 2022, 144, 57. DOI: 10.1021/jacs.1c10354
exo-BCN-NHS carbonate
CAS 1493802-77-9, Cat. No EN300-28319443
Synonyms: [(1R,8S,9R)-bicyclo[6.1.0]non-4-yn-9-yl]methyl 2,5-dioxopyrrolidin-1-yl carbonate; BCN-NHS; rel-((1R,8S,9r)-Bicyclo[6.1.0]non-4-yn-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate
Exo-BCN-NHS carbonate is a ready-to-use derivative of the well-known cyclooctyne, BCN-OH, EN300-378398, which is recognized for its balance of high reactivity and compact size.
The primary and most extensively studied reaction involving BCN derivatives is the Strain-Promoted Azide–Alkyne Cycloaddition (SPAAC).
They are also utilized in cycloadditions with tetrazines and nitrile imines.
- K. Lang, L. Davis, S. Wallace, M. Mahesh, D. J. Cox, M. L. Blackman, J. M. Fox and J. W. Chin, J. Am. Chem. Soc., 2012, 134, 10317. DOI: 10.1021/ja302832g
- X. Chen, F. Li and Y.-W. Wu, Chem. Commun., 2015, 51, 16537. DOI: 10.1039/C5CC05208D
endo-BCN-carbaldehyde
CAS 1426827-91-9, Cat. No EN300-46452871
Synonyms: (1R,8S,9S)-bicyclo[6.1.0]non-4-yne-9-carbaldehyde
Bicyclononyne with a carbonyl functionality is a derivative of the well-known cyclooctyne BCN-OH, EN300-342715. Endo-izomer.
- E. H. P. Leunissen, M. H. L. Meuleners, J. M. M. Verkade, J. Dommerholt, J. G. J. Hoenderop and F. L. van Delft, ChemBioChem, 2014, 15, 1446. DOI: 10.1002/cbic.201402030
exo-BCN-carbaldehyde
CAS 2166617-41-8, Cat. No EN300-46452840
Synonyms: (1R,8S,9R)-bicyclo[6.1.0]non-4-yne-9-carbaldehyde
Bicyclononyne with a carbonyl functionality is a derivative of the well-known cyclooctyne BCN-OH, EN300-378398. Exo-izomer.
- X. Li, Z. Liu and S. Dong, RSC Adv., 2017, 7, 44470. DOI: 10.1039/C7RA08136G
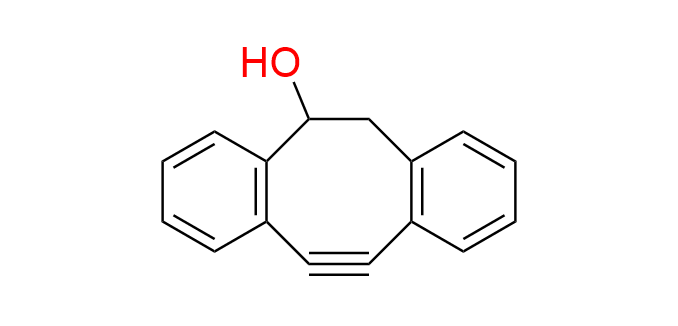
DIBO
CAS 1027338-06-2, Cat. No EN300-269065
Synonyms: DIBO-OH; tricyclo[10.4.0.0,4,9]hexadeca-1(16),4,6,8,12,14-hexaen-10-yn-2-ol; 3-Hydroxy-1,2:5,6-dibenzocyclooct-7-yne; 4-Dibenzocyclooctynol
4-Dibenzocyclooctynol (DIBO) is a well-known second-generation cyclooctyne. DIBO exhibits fast reaction rates in Strain-Promoted Azide–Alkyne Cycloaddition (SPAAC) and is among the most stable cyclooctynes. In addition to SPAAC, other studied reactions of DIBO include cycloadditions with nitrones and nitrile oxides.
- X. Ning, J. Guo, M. Wolfert and G.-J. Boons, Angew. Chem., Int. Ed., 2008, 47, 2253. DOI: 10.1002/anie.200705456
- M. F. Debets, S. S. van Berkel, J. Dommerholt, A. J. Dirks, F. P. J. T. Rutjes and F. L. van Delft, Acc. Chem. Res., 2011, 44, 805. DOI: 10.1021/ar200059z
- X. Ning, R. Temming, J. Dommerholt, J. Guo, D. Ania, M. Debets, M. Wolfert, G.-J. Boons and F. van Delft, Angew. Chem., Int. Ed., 2010, 49, 3065. DOI: 10.1002/anie.201000408
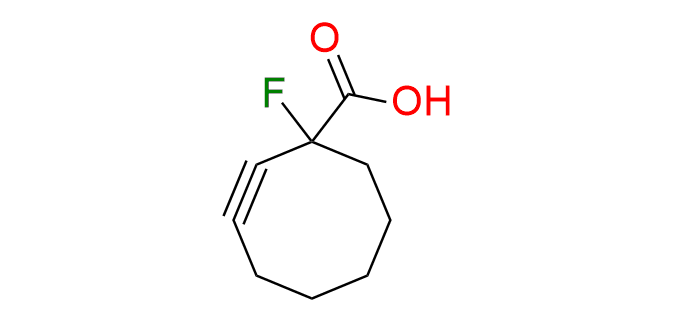
1-fluorocyclooct-2-yne-1-carboxylic acid
CAS 1227407-73-9; 2219376-60-8 (Li-salt), Cat. No EN300-1072052; EN300-1704293 (Li-salt)
1-Fluorocyclooct-2-yne-1-carboxylic acid is a simple second-generation monofluorocyclooctyne that exhibits moderate reactivity. Its main advantage lies in its minimal structural impact on the studied object.
- D. M. Beal, V. E. Albrow, G. Burslem, L. Hitchen, C. Fernandes, C. Lapthorn, L. R. Roberts, M. D. Selby and L. H. Jones, Org. Biomol. Chem., 2012, 10, 548. DOI: 10.1039/C1OB06398G
- L. Rong, C. Zhang, Q. Lei, H.-L. Sun, S.-Y. Qin, J. Feng and X.-Z. Zhang, Chem. Commun., 2015, 51, 388. DOI: 10.1039/C4CC08396B
- M. K. Schultz, S. G. Parameswarappa and F. C. Pigge, Org. Lett., 2010, 12, 2398. DOI: 10.1021/ol100774p
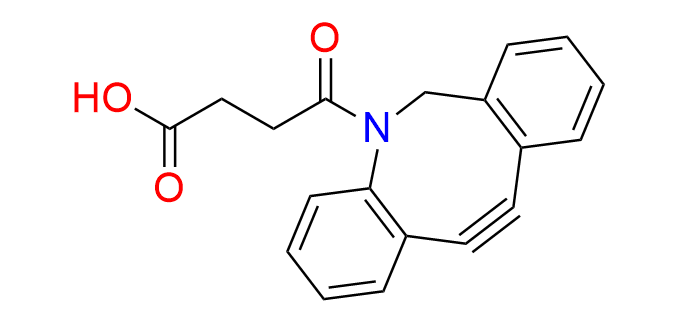
DBCO-Acid
CAS 1353016-70-2, Cat. No EN300-7393604
Synonyms: DIBAC-Acid; 4-{2-azatricyclo[10.4.0.0,4,9]hexadeca-1(16),4,6,8,12,14-hexaen-10-yn-2-yl}-4-oxobutanoic acid; DIBAC; ADIBO
Dibenzoazacyclooctyne (DBCO) is one of the most reactive (hetero)cyclooctynes in Strain-Promoted Azide–Alkyne Cycloaddition (SPAAC).
A butanoic acid handle is used for conjugation with the target molecule through simple amide coupling.
- L. S. Campbell-Verduyn, L. Mirfeizi, A. K. Schoonen, R. A. Dierckx, P. H. Elsinga and B. L. Feringa, Angew. Chem., Int. Ed., 2011, 50, 11117. DOI: 10.1002/anie.201105547
- S. Manabe, Y. Yamaguchi, K. Matsumoto, H. Fuchigami, T. Kawase, K. Hirose, A. Mitani, W. Sumiyoshi, T. Kinoshita, J. Abe, M. Yasunaga, Y. Matsumura and Y. Ito, Bioconjugate Chem., 2019, 30, 1343. DOI: 10.1021/acs.bioconjchem.9b00132
- H. Echigo, K. Mishiro, M. Munekane, T. Fuchigami, Y. Kitamura, S. Kinuya and K. Ogawa, Bioorg. Med. Chem., 2022, 70, 116919. DOI: 10.1016/j.bmc.2022.116919
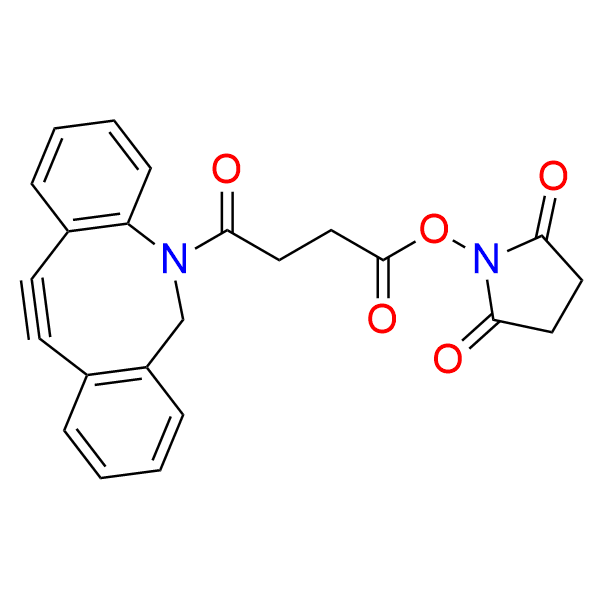
DBCO-NHS
CAS 1353016-71-3, Cat. No EN300-7398451
The N-hydroxysuccinimidyl ester of dibenzoazacyclooctyne (DBCO-NHS) is a ready-to-use derivative of DBCO, EN300-7393604.
It can be used for the direct acylation of the target molecule.
- L. S. Campbell-Verduyn, L. Mirfeizi, A. K. Schoonen, R. A. Dierckx, P. H. Elsinga and B. L. Feringa, Angew. Chem., Int. Ed., 2011, 50, 11117. DOI: 10.1002/anie.201105547
- H. E. Murrey, J. C. Judkins, C. W. am Ende, T. E. Ballard, Y. Fang, K. Riccardi, L. Di, E. R. Guilmette, J. W. Schwartz, J. M. Fox and D. S. Johnson, J. Am. Chem. Soc., 2015, 137, 11461. DOI: 10.1021/jacs.5b06847
- H. Echigo, K. Mishiro, M. Munekane, T. Fuchigami, Y. Kitamura, S. Kinuya and K. Ogawa, Bioorg. Med. Chem., 2022, 70, 116919. DOI: 10.1016/j.bmc.2022.116919
Can't find the substance you need? Contact us for custom synthesis solutions!