Tetrahedron 2015, 71 (8), 1311-1321
DOI: 10.1016/j.tet.2014.12.057
Nechayev M. A.; Gorobets N. Y.; Shishkina S. V.; Shishkin O. V.; Kovalenko S. M.
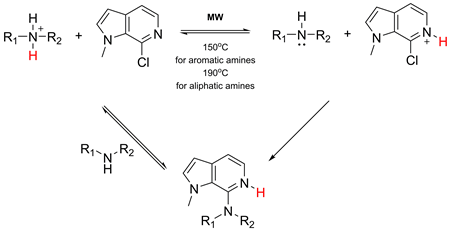
Derivatives of 7-amino-6-azaindole containing variable substituent in the amino group were synthesized via acid-catalyzed nucleophilic heteroaromatic substitution (SNHetarH+) using 7-chloro-6-azaindoles as substrates and aliphatic and aromatic amines as nucleophiles. The protonation of the pyridine nitrogen in the starting 7-chloro-6-azaindoles is presumed to be the key stage of the reaction mechanism discussed.