Tetrahedron 2014, 70 (18), 3011-3017
DOI: 10.1016/j.tet.2014.03.002
Yarmolchuk V. S.; Mykhalchuk V. L.; Mykhailiuk P. K.
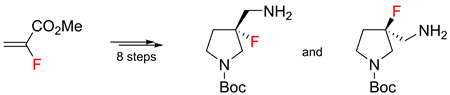
(R-)- and (S-)-3-fluoro-3-aminomethylpyrrolidines were synthesized from methyl α-fluoroacrylate in eight steps. α-Phenylethylamine was used as a chiral auxiliary to separate the racemic mixture. The overall synthesis yield was 31%.