Tetrahedron , 2011, 67 (5), 1030-1035
DOI: 10.1016/j.tet.2010.11.101
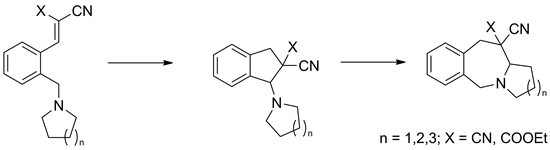
2-Cyano- and 2-carbethoxy-3-[2-(pyrrolidin-1-ylmethyl)phenyl]acrylonitriles were prepared through either amination of appropriate 3-[2-(bromomethyl)phenyl]acrylonitriles with pyrrolidine or condensation of 2-(pyrrolidin-1-ylmethyl)benzaldehyde with malononitrile and ethyl cyanoacetate. These acrylonitrile derivatives were shown to undergo easy mutual interconversion with 1-(pyrrolidin-1-yl)indane-2-carbonitriles driven by solvent polarity. Upon heating at 140–150 °C both acrylonitrile and indane derivatives were found to give 2,3,5,10,11,11a-hexahydro-1H-pyrrolo[1,2-b][2]benzazepine-11-carbonitriles. All transformations observed were rationalized in the terms of reactions related to the tert-amino effect. Furthermore, the corresponding piperidin-1-yl and azepan-1-yl analogs of the above acrylonitriles and indanes were obtained similarly. By analogy their heating afforded 1,2,3,4,6,11,12,12a-octahydropyrido[1,2-b][2]benzazepine-12-carbonitriles and 7,8,9,10,11,11a, 12,13-octahydro-5H-azepino[1,2-b][2]benzazepine-12-carbonitriles.
Gorulya A. P.; Tverdokhlebov A. V.; Tolmachev A. A.; Shishkin O. V.; Shishkina S. V.
Tetrahedron 2011, 67 (5), 1030-1035
DOI: 10.1016/j.tet.2010.11.101
