Tetrahedron Lett. , 2010, 51 (32), 4229-4232
DOI: 10.1016/j.tetlet.2010.06.032
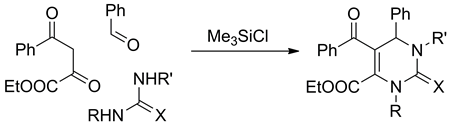
Chlorotrimethylsilane-promoted Biginelli-type reaction of ethyl 2,4-dioxo-4-phenylbutanoate, benzaldehyde, and various (thio)ureas is explored. The outcome of the reaction depends on the structure of the (thio)urea used and is strongly affected by the acceptor electronic properties of the COOEt substituent in the molecule of the starting β-dicarbonyl compound. The di- and tetrahydropyrimidine derivatives obtained possess two functional groups with orthogonal reactivity, and thus represent promising building blocks for drug discovery.
Ryabukhin S. V.; Plaskon A. S.; Bondarenko S. S.; Ostapchuk E. N.; Grygorenko O. O.; Shishkin O. V.; Tolmachev A. A.
Tetrahedron Lett. 2010, 51 (32), 4229-4232
DOI: 10.1016/j.tetlet.2010.06.032
