Eur. J. Med. Chem. , 2014, 85 245-254
DOI: 10.1016/j.ejmech.2014.07.103
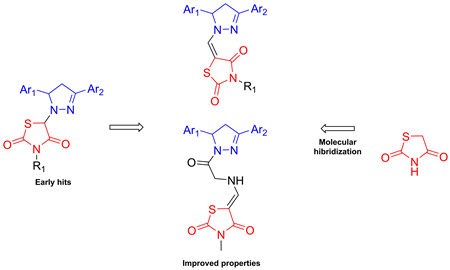
A series of novel 4-thiazolidinone–pyrazoline conjugates have been synthesized and tested for anti-Trypanosoma brucei activity. Screening data allowed us to identify five thiazolidinone–pyrazoline hybrids, which possess promising trypanocidal activity, with IC50 ≤ 1.2 μM. The highest active thiazolidinone–pyrazoline conjugates 3c and 6b (IC50 values of 0.6 μM and 0.7 μM, respectively) were 6-times more potent antitrypanosomal agents than nifurtimox. In addition, these compounds, as well as 6d and 6e had selectivity index higher than 50, and were more selective than nifurtimox. SAR study included substituent variations at the pyrazoline moiety, modifications of N3 position of the thiazolidinone portion, elongation of the linker between the heterocycles, as well as rhodanine–isorhodanine isomerism. It was also shown that methyl or aryl substitution at the thiazolidinone N3-position is crucial for trypanocidal activity.
Havrylyuk D.; Zimenkovsky B.; Karpenko O.; Grellier P.; Lesyk R.
Eur. J. Med. Chem. 2014, 85 245-254
DOI: 10.1016/j.ejmech.2014.07.103
