Synthesis , 2007, 12, 1811-1818
DOI: 10.1055/s-2007-983713
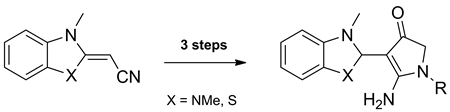
2-(1-Substituted-2-amino-4,5-dihydro-4-oxopyrrol-3-yl)-1,3-dimethylbenzimidazolium and -3-methylbenzothiazolium chlorides were prepared by reaction of 2-(2,3-dihydro-1,3-dimethylbenzimidazol-2-ylidene)- and 2-(3-methylbenzothiazol-2-ylidene)-4-chloro-3-oxobutanenitriles with primary amines. The salts obtained were reduced to 4-(2,3-dihydro-1,3-dimethylbenzimidazol-2-yl)- and 4-(2,3-dihydro-3-methylbenzothiazol-2-yl)-1-substituted-5-aminopyrrol-3(2H)-ones. These pyrrole derivatives were shown to serve as synthetic equivalents of the corresponding 2-aminopyrrole-3-carboxaldehydes. Thus, their treatment with phenylhydrazine yielded 1-substituted 2-amino-4,5-dihydro-4-oxopyrrole-3-carboxaldehyde phenylhydrazones, whereas condensation with malonodinitrile afforded 1-substituted 6-amino-2,3-dihydro-3-oxopyrrolo[2,3-b]pyridine-5-carbonitriles.
Tverdokhlebov A. V.; Denisenko A. V.; Tolmachev A. A.; Volovenko Y. M.
Synthesis 2007, 12, 1811-1818
DOI: 10.1055/s-2007-983713
