Synthesis , 2010, 06, 1009-1013
DOI: 10.1055/s-0029-1218641
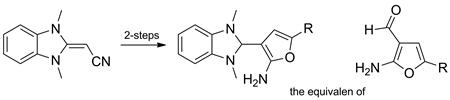
5′-Substituted 2-(2-amino-3-furanyl)-1,3-dimethyl-1H-benzimidazolium bromides were obtained in excellent yields upon heating (1,3-dimethyl-1,3-dihydro-2H-benzimidazol-2-ylidene)acetonitrile with α-bromo ketones in refluxing N,N-dimethylformamide. Reduction of the prepared quaternary salts with sodium borohydride afforded 5-substituted 3-(1,3-dimethyl-2,3-dihydro-1H-benzimidazol-2-yl)-2-furanamines. The latter were shown to be masked amino aldehydes. Thus, the corresponding 2-amino-3-furancarboxaldehyde phenylhydrazones and semicarbazones were obtained upon treatment with phenylhydrazine and semicarbazide, respectively, whereas condensation with malononitrile yielded 2-substituted 6-aminofuro[2,3-b]pyridine-5-carbonitriles.
Denisenko A. V.; Tverdokhlebov A. V.; Tolmachev A. A.; Volovenko Y. M.; Shishkina S. V.; Shishkin O. V.
Synthesis 2010, 06, 1009-1013
DOI: 10.1055/s-0029-1218641
