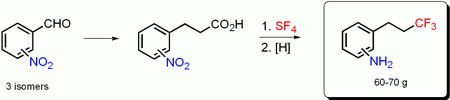J. Fluorine Chem. 2015, 171 174-176
DOI: 10.1016/j.jfluchem.2014.06.003
Trofymchuk S.; Bezdudny A.; Pustovit Y.; Mykhailiuk P. K.
Ortho-, meta- and para-isomers of (3,3,3-trifluoropropyl)aniline have been prepared in 60–70 g amount from the corresponding nitrobenzaldehydes in three steps. The key synthesis step was a transformation of the carboxylic group of 3-(nitrophenyl)propanoic acids into the trifluoromethyl group by SF4.