Synthesis , 2011, 02, 251-256
DOI: 10.1055/s-0030-1258371
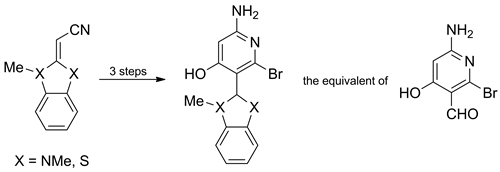
Acylation of (1,3-dimethylbenzimidazol-2-ylidene)-, (3-methylbenzothiazol-2-ylidene)-, and (3,4-dimethylthiazol-2-ylidene)-acetonitriles with 1-(cyanoacetyl)-3,5-dimethylpyrazole was found to proceed at the exocyclic carbon atom, yielding the appropriate 2-heterylidene-3-oxopentanedinitriles. Treatment of the prepared dinitriles with hydrobromic acid in refluxing acetic acid afforded 2-(6-amino-2-bromo-4-hydroxypyridin-3-yl)-substituted quaternary salts of benzimidazolium, benzothiazolium, and thiazolium. Their structures were confirmed unambiguously by X-ray crystallographic studies. Reduction of these quaternary salts with excess sodium borohydride yielded the corresponding dihydro (in the case of benzoazoles) or tetrahydro (in the case of thiazole) derivatives, which were shown to be synthetic equivalents of the title nicotinaldehyde.
Denisenko A. V.; Tverdokhlebov A. V.; Tolmachev A. A.; Volovenko Y. M.; Shishkina S. V.; Shishkin O. V.
Synthesis 2011, 02, 251-256
DOI: 10.1055/s-0030-1258371
