Eur. J. Org. Chem. 2015, 29, 6466-6471
DOI: 10.1002/ejoc.201500746
Chernykh A. V.; Radchenko D. S.; Chernykh A. V.; Kondratov I. S.; Tolmachova N. A.; Datsenko O. P.; Kurkunov M. A.; Zozulya S. X.; Kheylik Y. P.; Bartels K.; Daniliuc C. G.; Haufe G.
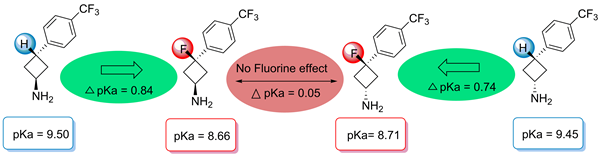
Hitherto unknown cis- and trans-3-alkyl- and 3-aryl-3-fluorocycobutylamines have been synthesised selectively from 3-oxocyclobutane carboxylic acid in six or seven steps. Comparison of their pKa and log D values with those of the fluorine-free parent compounds showed acidification by about 0.8 units, irrespective of the stereochemistry. This indicates that there are no through-space interactions between fluorine and the amino function – a conclusion that was supported by the results of X-ray analysis. Fluorinated trans-compounds were found to be more lipophilic (Δ log P ≈ 1) compared with the non-fluorinated analogues, whereas the difference was marginal for cis isomers.