J. Enzyme Inhib. Med. Chem. 2020, 35 (1), 306‑310
DOI: 10.1080/14756366.2019.1698562
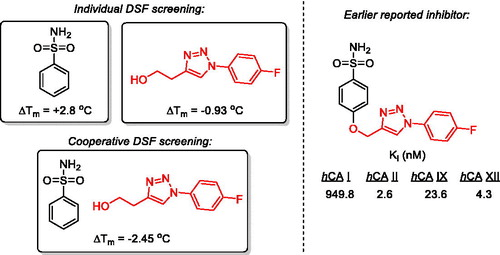
The differential scanning fluorimetry (DSF) screening of 5.692 fragments in combination with benzenesulfonamide (BSA) against bovine carbonic anhydrase (bCA) delivered >100 hits that either caused, on their own, a significant thermal shift (ΔTm, °C) in the protein melting temperature or significantly influenced the thermal shift observed for BSA alone. Three hits based on 1,2,3-triazole moiety represent the periphery of the recently reported potent inhibitors of hCA II, IX and XII which were efficacious in vivo. Such a re-discovery of suitable BSA periphery essentially validates the new fragment-based approach to the discovery of future CAIs. Structures of other validated fragment hits are reported.