Eur. J. Org. Chem. 2019, (34), 5937-5949
DOI: 10.1002/ejoc.201901001
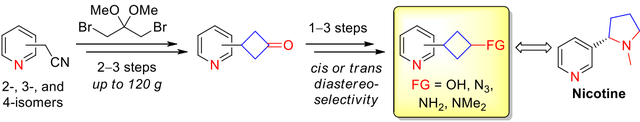
An approach to all isomeric 3‐pyridylcyclobutane‐derived building blocks, i.e. ketones, alcohols and amines, is described. Synthesis of the title compounds relied on the five‐step reaction sequence including alkylation of isomeric pyridyl acetonitriles with 1,3‐dibromo‐2,2‐dimethoxypropane. Hydrolysis, decarboxylation and removal of the ketal moiety led to the key 3‐pyridylcyclobutanones (obtained on up to 120 g scale in a single run), which were transformed into the corresponding alcohols and amines with high diastereoselectivity. The title cyclobutanone derivatives were used to synthesize three isomeric nicotine analogues, as well as for parallel synthesis of a small lead‐like compound library via reductive amination.