Synthesis , 2012, 44 (06), 895-902
DOI: 10.1055/s-0031-1289733
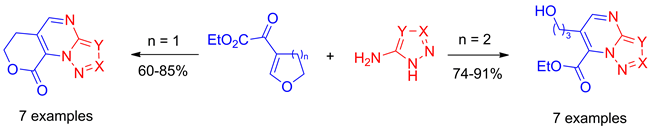
The reactions of ethyl (4,5-dihydrofuran-3-yl)-2-oxoacetate and ethyl 2-(3,4-dihydro-2H-pyran-6-yl)-2-oxoacetate, as 1,3-bielectrophiles, with N-unsubstituted 5-aminoazoles, as N-C-N-binucleophiles, are the subject of this work. Regioselective heterocyclizations of ethyl 2-(3,4-dihydro-2H-pyran-6-yl)-2-oxoacetate lead to 3-hydroxypropyl-7-ethoxycarbonyl substituted pyrazolo[1,5-a]pyrimidines and triazolo[1,5-a]pyrimidines whilst the corresponding reactions of ethyl (4,5-dihydrofuran-3-yl)-2-oxoacetate result in the formation of oxodihydropyrano[4,3-e] annulated products.
Stepaniuk O. O.; Matvienko V. O.; Kondratov I. S.; Shishkin O. V.; Volochnyuk D. M.; Mykhailiuk P. K.; Tolmachev A. A.
Synthesis 2012, 44 (06), 895-902
DOI: 10.1055/s-0031-1289733
