J. Heterocycl. Chem. , 2013, 50 (6), 1299-1303
DOI: 10.1002/jhet.1568
Ostapchuk E. N.; Plaskon A. S.; Grygorenko O. O.; Tolmachev A. A.; Ryabukhin S. V.
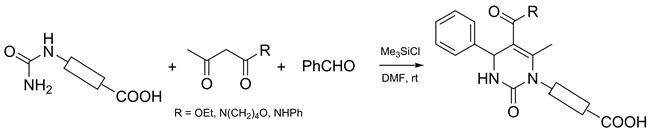
Chlorotrimethylsilane-promoted Biginelli-type reaction of benzaldehyde, acetoacetic acid derivatives, and various carboxyl-containing ureas was explored. It was found that the steric load of the urea substituents influenced strongly the reaction outcome; in particular, the method was efficient only in the case of unbranched mono-substituted ureas bearing either aliphatic or aromatic groups. The method allows performing a one-pot, protecting group free synthesis of dihydropyrimidines possessing carboxylic functionality.
Ostapchuk E. N.; Plaskon A. S.; Grygorenko O. O.; Tolmachev A. A.; Ryabukhin S. V.
J. Heterocycl. Chem. 2013, 50 (6), 1299-1303
DOI: 10.1002/jhet.1568
