Eur. J. Med. Chem. , 2014, 84 160-172
DOI: 10.1016/j.ejmech.2014.07.023
A novel series of compounds containing a polar, non-flat 2-imidazoline core was designed based on the SAR information available for aromatic azole cyclooxygenase-2 inhibitors. While the majority of the compounds prepared using an earlier developed imidazoline N-arylation methodology turned out to be inferior to the known COX-2 inhibitors, one lead compound displayed potency (300 nM) comparable to clinically used Celecoxib and was shown to be more selective. The series represents the first example of selective COX-2 inhibitors built around a distinctly polar core, contradicting an earlier accepted view that a lipophilic scaffold is required for high inhibitor potency. The lead compound demonstrated very good oral bioavailability in mice, slow metabolic degradation, modest distribution into the brain and a remarkable anti-inflammatory efficacy in carrageenan-induced mouse paw edema model. A foundation has therefore been laid for a chemically novel series of COX-2 inhibitors that has a potential for diverse therapeutic applications in inflammatory disease area.
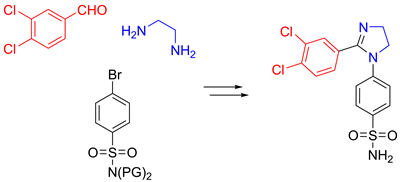
Sarnpitak P.; Mujumdar P.; Morisseau C.; Hwang S. H.; Hammock B.; Iurchenko V.; Zozulya S.; Gavalas A.; Geronikaki A.; Ivanenkov Y.; Krasavin M.
Eur. J. Med. Chem. 2014, 84 160-172
DOI: 10.1016/j.ejmech.2014.07.023
