ACS Comb. Sci. 2015, 17 (6), 348-354
DOI: 10.1021/acscombsci.5b00024
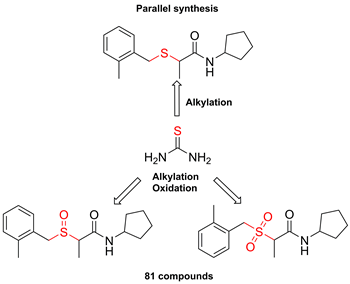
A simple and cost-effective one-pot parallel synthesis approach to sulfides, sulfoxides, and sulfones from thiourea was elaborated. The method combines two procedures optimized to the parallel synthesis conditions: alkylation of thiourea with alkyl chlorides and mono or full oxidation of in situ generated sulfides with H2O2 or H2O2-(NH4)2MoO4. The experimental set up required commonly used lab equipment: conventional oven and ultrasonic bath; the work up includes filtration or extraction with chloroform. The method was evaluated on an 81 member library of drug-like sulfides, sulfoxides, and sulfones yielding the compounds on a 30-300 mg scale. A small-scale synthesis of 2-(benzhydrylsulfinyl)acetamide (modafinil) utilizing our approach resulted in similar efficiency to the published procedures.