Synthesis , 2014, 46 (13), 1765-1772
DOI: 10.1055/s-0033-1341226
Bogolubsky A. V.; Moroz Y. S.; Pipko S. E.; Panov D. M.; Konovets A. I.; Doroschuk R.; Tolmachev A.
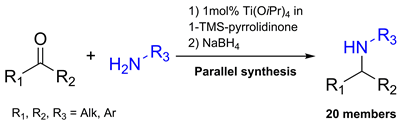
A combination of a condensation mixture [a Lewis acid, Ti(Oi-Pr)4, and a water scavenger, 1-(trimethylsilyl)-2-pyrrolidinone] and a simple reductant (NaBH4) provides an efficient one-pot approach to parallel synthesis of secondary amines by the reductive amination of ketones. The approach demonstrated its applicability to a variety of substrates with different degree of hindrance of an amino or a carbonyl group affording products in moderate to high yields.
Bogolubsky A. V.; Moroz Y. S.; Pipko S. E.; Panov D. M.; Konovets A. I.; Doroschuk R.; Tolmachev A.
Synthesis 2014, 46 (13), 1765-1772
DOI: 10.1055/s-0033-1341226
