Chem. Heterocycl. Compd. 2015, 51 (4), 310‑319
DOI: 10.1007/s10593-015-1700-y
Petrova O. N.; Zamigajlo L.; Ostras K.; Shishkina S. V.; Shishkin O. V.; Borisov A. V.; Musatov V. I.; Shirobokova M. G.; Lipson V. V.
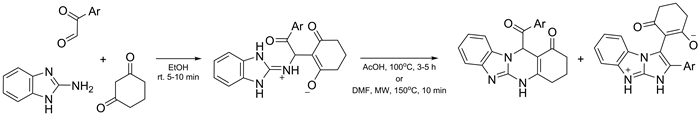
Three-component reactions of 2-aminobenzimidazole with arylglyoxals and 1,3-cyclohexanedione have been studied under conventional heating and microwave irradiation. Different product types including the Michael adduct and fused heterocyclic systems were obtained. Conditions for the selective formation of 12-aroylbenzimidazo[2,1-b]quinazolin-1(2H)-ones and 3-oxo-2-(2-aryl-1H-imidazo[1,2-a]-benzimidazol-9-ium-3-yl)cyclohex-1-enolates have been determined. The structures of all compound types were established by an X-ray diffraction study.