Org. Lett. , 2012, 14 (20), 5254-5257
DOI: 10.1021/ol302412a
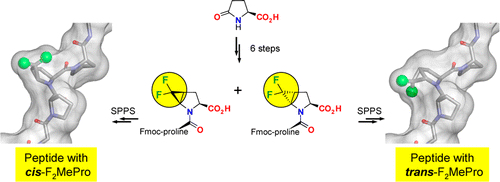
Substituted prolines exert diverse effects on the backbone conformation of proteins. Novel difluoro-analogues were obtained by adding difluorocarbene to N-Boc-4,5-dehydroproline methyl ester, which gave the trans-adduct as the sole product with 71% yield. Upon cleavage of the N-protection group the free amino acid decomposed rapidly. Its incorporation into the proline-rich cell-penetrating "sweet arrow peptide" was thus accomplished using a dipeptide strategy. Two building blocks, containing either cis- or trans-4,5-difluoromethanoproline, were obtained by difluorocyclopropanation of the aminoacyl derivatives of 4,5-dehydroproline. The resulting dipeptides were stable under standard conditions of Fmoc solid phase peptide synthesis and, thus, suitable to study conformational effects.
Kubyshkin V. S.; Mykhailiuk P. K.; Afonin S.; Ulrich A. S.; Komarov I. V.
Org. Lett. 2012, 14 (20), 5254-5257
DOI: 10.1021/ol302412a
