Chem. Heterocycl. Compd. 2019, 55 (4-5), 421-434
DOI: 10.1007/s10593-019-02475-9
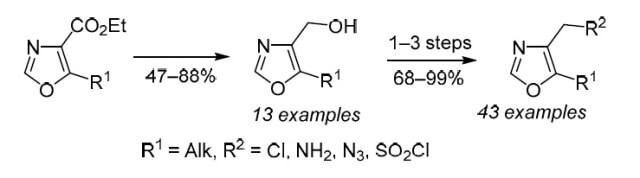
An efficient approach to the preparation of novel sp3-enriched 4,5-disubstituted oxazoles bearing a functional group at the C-4 position is described. The method commenced with synthesis of ethyl oxazole-4-carboxylates (13 examples, 63–99% yield), with subsequent function insertion to the heterocyclic core by late-stage functional group transformation. The LiBH4-mediated reduction of ethyl oxazole-4-carboxylates was the only method which could be optimized at multigram scale (up to 40 g), and its scope was demonstrated by preparation of 13 alcohols with (cyclo)alkyl, fluoroalkyl, or N-Boc-aminoalkyl moiety at the C-5 position (47–89% yield). The utility of these key intermediates was demonstrated by the preparation of chlorides (13 examples, 90–99% yield), azides (13 examples, 83–99% yield), amines (13 examples, 80–98% yield), and sulfonyl chlorides (4 examples, 68–97% yield) – advanced building blocks for synthetic and medicinal chemistry.