Eur. J. Org. Chem. , 2014, 34, 7692-7698
DOI: 10.1002/ejoc.201403013
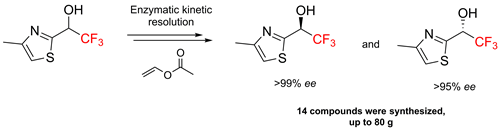
A convenient approach towards enantiopure (R) and (S) isomers of 2,2,2-trifluoro-1-(heteroaryl)ethanols was developed. The enzyme-catalyzed kinetic resolution of the corresponding racemic mixtures was achieved by using a two-step protocol that involved an acylation and a hydrolysis step in the presence of Burkholderia cepacia and CAL-B lipase (Candida antarctica lipase B), respectively. Fourteen compounds were resolved on a multigram scale by using this approach. The observed enantioselectivities correlated well with the results of molecular docking experiments.
Kucher O. V.; Kolodiazhnaya A. O.; Smolii O. B.; Prisuazhnyk D. V.; Tolmacheva K. A.; Zaporozhets O. A.; Moroz Y. S.; Mykhailiuk P. K.; Tolmachev A. A.
Eur. J. Org. Chem. 2014, 34, 7692-7698
DOI: 10.1002/ejoc.201403013
