Mol. Diver. , 2012, 16 (4), 625-637
DOI: 10.1007/s11030-012-9407-9
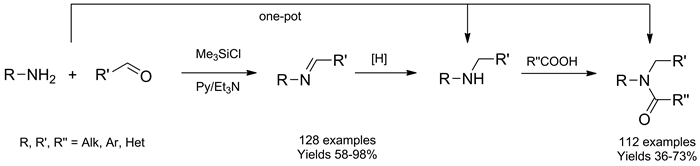
128 Azomethines were synthesized through condensation of carbonyl compounds with various amines in pyridine in the presence of Me3SiCl as promoter and water scavenger in 58–98 % yield. Et3N was added to reaction mixtures before precipitating the product with H2O to prevent acid catalyzed hydrolysis of the C=N bond. The scope and limitation of the method are discussed. High yields and simple setup/workup procedure make this method suitable for the combinatorial synthesis of azomethines, which are suitable as starting materials for high throughput synthesis of various combinatorial libraries. The azomethines synthesized were used as starting materials in a one-pot combinatorial synthesis of amines and amides.
Ryabukhin S.; Panov D.; Plaskon A.; Chuprina A.; Pipko S.; Tolmachev A.; Shivanyuk A.
Mol. Diver. 2012, 16 (4), 625-637
DOI: 10.1007/s11030-012-9407-9
