J. Heterocycl. Chem. , 2012, 49 (5), 1147-1150
DOI: 10.1002/jhet.972
Ryabukhin S. V.; Naumchik V. S.; Grygorenko O. O.; Tolmachev A. A.
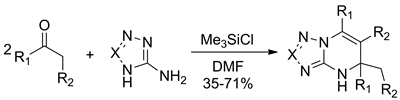
Chlorotrimethylsilane-promoted reaction of ketones and aminoazoles (i.e., 3-amino-1,2,4-triazoles, 5-aminotetrazole) at 2 : 1 ratio resulted in the formation of 4,5-dihydroazolo[1,5-a]pyrimidine derivatives as the single regioisomers in 35 – 71% yields. In the case of tert-butylmethylketone and 5-aminotetrazole as the starting materials, the solvent (dimethylformamide) entered the reaction instead of the second ketone molecule.
Ryabukhin S. V.; Naumchik V. S.; Grygorenko O. O.; Tolmachev A. A.
J. Heterocycl. Chem. 2012, 49 (5), 1147-1150
DOI: 10.1002/jhet.972
