J. Comb. Chem. , 2010, 12 (4), 510-517
DOI: 10.1021/cc100040q
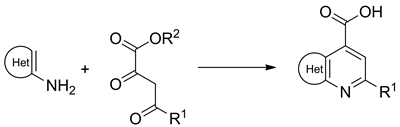
A library of fused pyridine-4-carboxylic acids (including pyrazolo[3,4-b]pyridines, isoxazolo[5,4-b]pyridines, furo[2,3-b]pyridines, thieno[2,3-b]pyridines, and pyrido[2,3-d]pyrimidines) was generated by Combes-type reaction of acyl pyruvates and electron-rich amino heterocycles followed by hydrolysis of the ester. The library members were also demonstrated to undergo the standard combinatorial transformations including amide coupling and esterification, as well as less common heterocyclizations to 1,2,4-triazoles and 1,2,4-oxadiazoles.
Volochnyuk D. M.; Ryabukhin S. V.; Plaskon A. S.; Dmytriv Y. V.; Grygorenko O. O.; Mykhailiuk P. K.; Krotko D. G.; Pushechnikov A.; Tolmachev A. A.
J. Comb. Chem. 2010, 12 (4), 510-517
DOI: 10.1021/cc100040q
