ACS Comb. Sci. , 2012, 14 (12), 631-635
DOI: 10.1021/co300082t
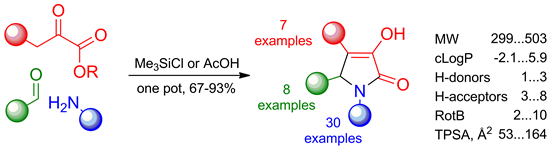
A convenient procedure for the parallel synthesis of 3-hydroxy-1,5-dihydro-2H-pyrrol-2-ones through a three-component condensation of active methylene compounds, aldehydes, and amines was developed. It was shown that the use of acetic acid as the reaction medium was suitable for the considerably reactive substrates with no additional functionalities. The substrates with low reactivity and those possessing carboxylic groups or additional basic centers required the use of DMF as the solvent and chlorotrimethylsilane as the reaction promoter was necessary. More than 3000 pyrrolones were synthesized by the developed procedure. To demonstrate the scope of the described approach 114 library representatives were fully characterized.
Ryabukhin S. V.; Panov D. M.; Plaskon A. S.; Grygorenko O. O.
ACS Comb. Sci. 2012, 14 (12), 631-635
DOI: 10.1021/co300082t
