Eur. J. Org. Chem. 2020, 2020 (23), 3367-3377
DOI: 10.1002/ejoc.202000078
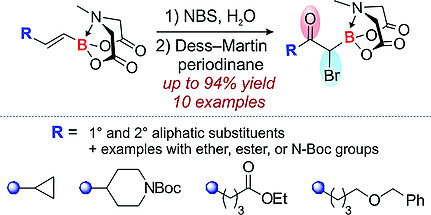
A protocol for the preparation of α‐boryl‐α‐bromoketones from alkenyl MIDA boronates was developed and applied to functionalized aliphatic derivatives. The reaction sequence included regioselective hydroxybromination of olefin moiety, followed by oxidation of alcohol group with Dess–Martin periodinane. The target trifunctional boronate‐containing derivatives were obtained in up to 94 % yield over two steps starting from alkenyl MIDA boronates. In some cases, functional groups present in the substrate participated in the bromohydroxylation step via intramolecular nucleophilic attack at the bromonium cation leading to cyclic products. Additionally, the reactivity of aliphatic α‐boryl‐α‐bromoketones was illustrated by nucleophilic substitution at the α‐C atom and heterocyclization reactions.