Mol. Diver. , 2013, 17 (3), 471-477
DOI: 10.1007/s11030-013-9444-z
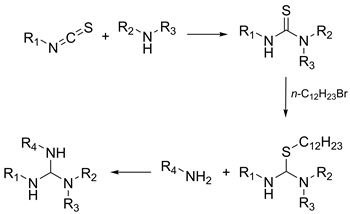
An efficient solution-phase parallel synthesis of alkylated guanidines from commercial thioisocyanates and amines is described. In the first step, a thioisocyanate reacts with one equivalent of ammonia or a primary or secondary amine to give a thiourea intermediate. The latter is S-alkylated with n-dodecyl bromide resulting in the corresponding thiouronium bromide. Finally, the reaction of the thiouronium salt with a second equivalent of ammonia or a primary amine yields an alkylated guanidine. All three synthetic steps are easily combined in a one-pot high-yielding procedure with a simple work-up. Ca. 250 guanidine derivatives with high structural and functional diversity were synthesized by the developed method. 35 representatives reported in this study were fully characterized
Bogolubsky A. V.; Grishchenko A.; Pipko S. E.; Konovets A.; Chuprina A.; Tolmachev A.; Boyko A. N.; Chekotylo A.; Lukin O.
Mol. Diver. 2013, 17 (3), 471-477
DOI: 10.1007/s11030-013-9444-z
