J. Heterocycl. Chem. , 2013, 50 (5), 1071-1077
DOI: 10.1002/jhet.1029
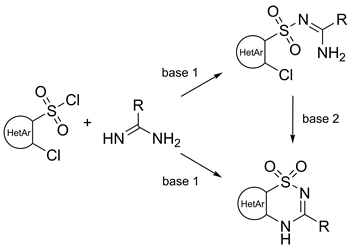
A set of synthetic procedures was developed to yield functionalized pyrido-, pyrimido-, and thiazo-annulated thiadiazine-1,1-dioxides on a preparative scale. In all cases the thiadiazine-1,1-dioxide ring closure was carried out through a reaction of hetaryl-sulfonyl chlorides with amidines under mild noncatalytic conditions. In the case of 2-chloropyridine-3-sulfonyl chloride derivatives and 2,4-dichlorothiazole-5-sulfonyl chloride open-chain sulfonylated amidine intermediates were isolated and then subjected to the cyclization step. The reaction with 2,4-dichloropyrimidine-5-sulfonyl chloride gave rise to the corresponding thiadiazine-1,1-dioxides in one-pot. Similarly, a reaction of 2-chloropyridine-3-sulfonamide with lactime ethers proceeded in one-pot readily giving the corresponding thiadiazine-1,1-dioxides. Remaining chlorine atoms on the prepared hetaryl-annulated benzothiadiazine-1,1-dioxides readily undergo aromatic nucleophilic displacement reactions serving thus as additional variation points for the design of biologically potent compounds.
Cherepakha A.; Kovtunenko V. O.; Tolmachev A.; Lukin O.
J. Heterocycl. Chem. 2013, 50 (5), 1071-1077
DOI: 10.1002/jhet.1029
