Synthesis , 2012, 44 (13), 1974-1976
DOI: 10.1055/s-0031-1290295
Trofymchuk S.; Bezdudny A. V.; Pustovit Y. M.; Lukin O.; Boyko A. N.; Chekotylo A.; Tolmachev A. A.; Mykhailiuk P. K.
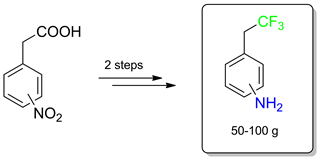
Three isomers of C-(2,2,2-trifluoroethyl)aniline were prepared on a multigram scale from readily available nitrophenylacetic acids in two steps. First, the carboxy groups of the latter were converted into the trifluoromethyl moieties by treatment with sulfur tetrafluoride. The obtained 2,2,2-trifluoroethyl-substituted nitrobenzenes were reduced catalytically into the corresponding anilines.
Trofymchuk S.; Bezdudny A. V.; Pustovit Y. M.; Lukin O.; Boyko A. N.; Chekotylo A.; Tolmachev A. A.; Mykhailiuk P. K.
Synthesis 2012, 44 (13), 1974-1976
DOI: 10.1055/s-0031-1290295
