Eur. J. Org. Chem. 2015, 11, 2482-2491
DOI: 10.1002/ejoc.201500032
Kondratov I. S.; Tolmachova N. A.; Dolovanyuk V. G.; Gerus I. I.; Daniliuc C.-G.; Haufe G.
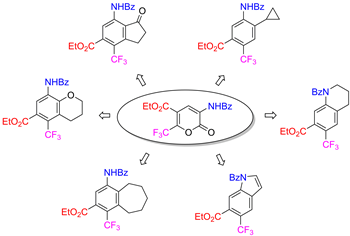
Diels–Alder reactions of ethyl 3-benzamido-2-oxo-6-(trifluoromethyl)-2H-pyran-5-carboxylate (1a) with electronically different dienophiles, including cyclic enol ethers, cycloalkenes, α,β-unsaturated ketones, and terminal acetylenes, are useful for the efficient and selective three-step preparation of trifluoromethyl-containing aromatic compounds such as 3-aminobenzoic acid derivatives. We presume that the presence of the trifluoromethyl group is the main factor in determining the regioselectivity of the initial cycloaddition.