Eur. J. Org. Chem. , 2013, 14, 2891-2897
DOI: 10.1002/ejoc.201300030
Iminov R. T.; Mashkov A. V.; Chalyk B. A.; Mykhailiuk P. K.; Tverdokhlebov A. V.; Tolmachev A. A.; Volovenko Y. M.; Shishkin O. V.; Shishkina S. V.
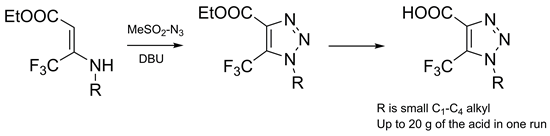
The reaction of ethyl 3-(alkylamino)-4,4,4-trifluoro-but-2-enoates with mesyl azide in the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) gave ethyl 1-alkyl-5-trifluoromethyl-1,2,3-triazole-4-carboxylates in good yields. Further hydrolysis of the ester group afforded the title compounds on a multigram scale.
Iminov R. T.; Mashkov A. V.; Chalyk B. A.; Mykhailiuk P. K.; Tverdokhlebov A. V.; Tolmachev A. A.; Volovenko Y. M.; Shishkin O. V.; Shishkina S. V.
Eur. J. Org. Chem. 2013, 14, 2891-2897
DOI: 10.1002/ejoc.201300030
