Synthesis , 2012, 44 (06), 903-908
DOI: 10.1055/s-0031-1289702
Tkachenko A. N.; Radchenko D. S.; Mykhailiuk P. K.; Shishkin O. V.; Tolmachev A. A.; Komarov I. V.
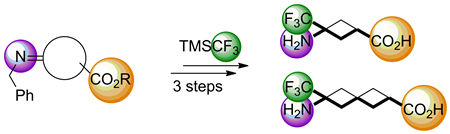
Straightforward gram-scale syntheses of a novel γ-trifluoromethyl γ-amino acid and a novel ε-trifluoromethyl-ε-amino acid are described. The key step in both syntheses is an acid-catalyzed nucleophilic trifluoromethylation of a cyclic N-benzylimine possessing an ester group by using the Ruppert--Prakash reagent [trimethyl(trifluoromethyl)silane]. The strategy provides a potentially general approach for the synthesis of x-trifluoromethyl x-amino acids.
Tkachenko A. N.; Radchenko D. S.; Mykhailiuk P. K.; Shishkin O. V.; Tolmachev A. A.; Komarov I. V.
Synthesis 2012, 44 (06), 903-908
DOI: 10.1055/s-0031-1289702
