Mol. Diver. , 2011, 15 (1), 189-195
DOI: 10.1007/s11030-010-9253-6
Ryabukhin S.; Plaskon A.; Boron S.; Volochnyuk D.; Tolmachev A.
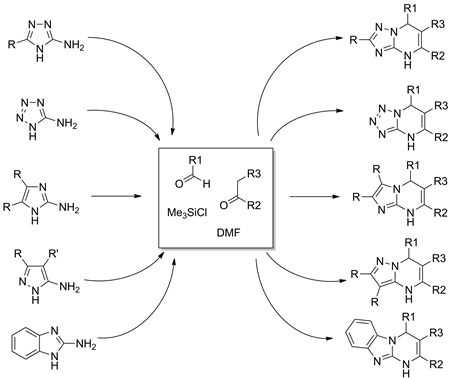
Chlorotrimethylsilane (TMSCl) has been utilized as an efficient promoter and water scavenger in the synthesis of diverse dihydropyrimidines via Biginelli type MCR-heterocyclization using aminoheterocycles. High yields and a simple workup of target compounds enable the facile generation of combinatorial libraries comprising more than 2 000 compounds of high structural and functional diversity. A representative set of 89 compounds is described.
Ryabukhin S.; Plaskon A.; Boron S.; Volochnyuk D.; Tolmachev A.
Mol. Diver. 2011, 15 (1), 189-195
DOI: 10.1007/s11030-010-9253-6
