J. Fluorine Chem. , 2010, 131 (2), 238-247
DOI: 10.1016/j.jfluchem.2009.10.019
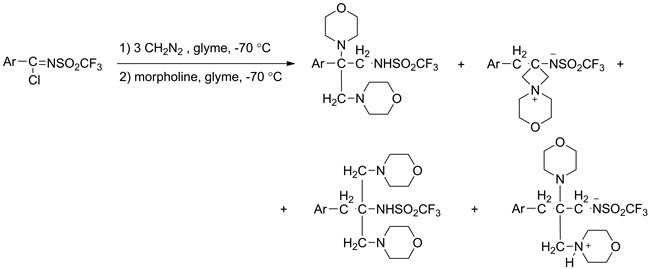
The aza analogues of carboxylic acids chlorides containing the =NSO2CF3 and =NSO2CH3 groups instead of oxygen atom were used in the Arndt–Eistert reaction. It was found that N-trifluoromethylsulfonyl-(4-fluorophenyl)-carboximidoyl chloride 1 reacts with diazomethane vigorously even at −70 °C with formation of 1-trifluoromethylsulfonylamino-2-(4-fluorophenyl)-2,3-dimorpholine-4-yl-propane 3, 2-trifluoromethylsulfonylamino-2-(4-fluorobenzyl)-7-oxa-4-azonia-spiro[3.5]nonane 4, 2-trifluoromethylsulfonylamino-2-(4-fluorobenzyl)-1,3-dimorpholine-4-yl-propane 5 and 1-trifluoromethylsulfonylamino-2-(4-fluorobenzyl)-2,3-dimorpholine-4-yl-propane 6. Reaction of N-methylsulfonylbenzcarboximidoyl chloride 8 with diazomethane proceeds at −15 °C yielding 4-chloro-4-methylsulfonylaminomethyl-3-phenyl-4,5-dihydro-1H-pyrazoline 9.
Yagupolskii L. M.; Maletina I. I.; Sokolenko L. V.; Vlasenko Y. G.; Drozdova M. V.; Polovinko V. V.
J. Fluorine Chem. 2010, 131 (2), 238-247
DOI: 10.1016/j.jfluchem.2009.10.019
