Synthesis , 2011, 01, 133-141
DOI: 10.1055/s-0030-1258339
Ostrovskyi D.; Iaroshenko V. O.; Ali I.; Mkrtchyan S.; Villinger A.; Tolmachev A.; Langer P.
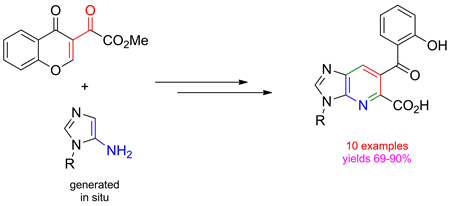
The reaction of 5-amino-1H-imidazoles with 3-methoxalylchromone provided a set of imidazo[4,5-b]pyridines (1-desazapurines) bearing the CO2Me substituent at the α-position of the pyridine core. The corresponding acids were synthesized by subsequent hydrolysis of the ester function. Being typical purine isosteres, 1-desazapurines are considered to be potent pharmacophores, and are widely used in drug design and medicinal chemistry.
Ostrovskyi D.; Iaroshenko V. O.; Ali I.; Mkrtchyan S.; Villinger A.; Tolmachev A.; Langer P.
Synthesis 2011, 01, 133-141
DOI: 10.1055/s-0030-1258339
