Org. Biomol. Chem. , 2010, 8 (23), 5280-5284
DOI: 10.1039/C0OB00379D
Mkrtchyan S.; Iaroshenko V. O.; Dudkin S.; Gevorgyan A.; Vilches-Herrera M.; Ghazaryan G.; Volochnyuk D. M.; Ostrovskyi D.; Ahmed Z.; Villinger A.; Sosnovskikh V. Y.; Langer P.
The first synthesis of 3-methoxalylchromone was described. The reaction of the latter with electron-rich aminoheterocycles afforded a set of heteroannelated pyridines bearing a CO2Me substituent located at the α-position of the pyridine core.
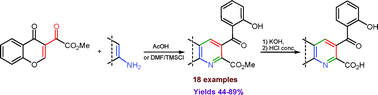
Mkrtchyan S.; Iaroshenko V. O.; Dudkin S.; Gevorgyan A.; Vilches-Herrera M.; Ghazaryan G.; Volochnyuk D. M.; Ostrovskyi D.; Ahmed Z.; Villinger A.; Sosnovskikh V. Y.; Langer P.
Org. Biomol. Chem. 2010, 8 (23), 5280-5284
DOI: 10.1039/C0OB00379D
