Chemistry 2023, 29 (70), e202302454
DOI: 10.1002/chem.202302454
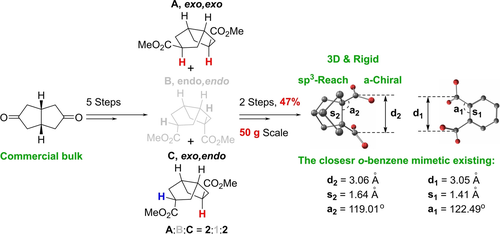
Herein, we present previously unavailable C(sp3)-rich polycyclic hydrocarbon scaffolds that have the potential to become valuable tools in medicinal chemistry and crop science as saturated bioisosteres of benzenoids. We have developed a scalable protocol (up to 50 g from a single synthetic run) for the synthesis of tricyclo[3.3.0.03,7]octane (bisnoradamantane or stellane) 1,5-dicarboxylic acid derivatives. X-ray crystallographic analysis of the stellane 1,5-dicarboxylic acid dimethyl ester has revealed that this scaffold is an optimal saturated isostere for ortho-disubstituted benzene where substituents exhibit in-plane topology. The synthetic protocol is based on the oxidative cyclization of dimethyl octahydropentalene-2,5-dicarboxylate (DMOD) through lithiation followed by I2 oxidation. The reaction outcome is determined by the stereochemistry of the substrate. While the endo,endo cis-DMOD, exclusively gives the “unwanted” Claisen cyclization product, the exo,endo cis- and exo,exo cis- stereoisomers afford the desired stellane 1,5-dicarboxylic acid dimethyl ester quantitatively. DFT computations have revealed that the reaction proceeds via the dianion of dimethyl octahydropentalene-2,5-dicarboxylate, which undergoes SET oxidation by I2 to form a radical anion. The subsequent cyclization followed by a second SET oxidation gives the desired stellane derivative.