ChemistrySelect 2020, 5 (43), 13569-13574
DOI: 10.1002/slct.202003500
Cherednichenko A.; Bezgubenko L.; Rusanov E.; Onys'ko P.; Rassukana Y.
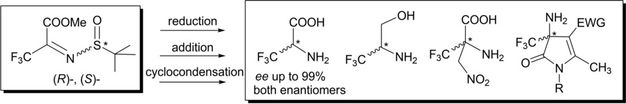
A convenient synthetic method for (R)- and (S)-N-tert-butylsulfinyl imines of methyl trifluoropyruvate has been developed. The synthetic potentialities of these chiral building blocks for the preparation of nonracemic trifluoroalanines, trifluoromethylated amino alcohols, β-nitro-α-amino acids, and pyrrolinone carboxylic acid derivatives have been demonstrated.