Chem. Heterocycl. Compd. 2020, 56 (3), 320-325
DOI: 10.1007/s10593-020-02662-z
Ivonin S.; Rusanov E. B.; Dmitriy V.
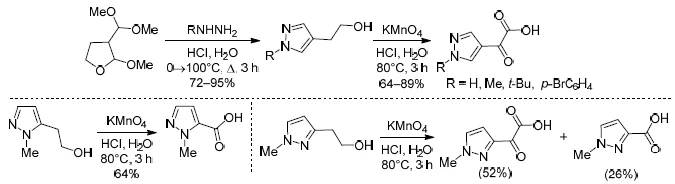
An efficient approach to the preparation of N-substituted 2-(pyrazol-4-yl)ethanols based on recyclization reaction of 3-(dimethoxymethyl)-2-methoxytetrahydrofuran with hydrazines is described. Oxidation by KMnO4 led to 2-(pyrazol-4-yl)-2-oxoacetic acids. In contrast, 2-(pyrazol-5-yl)ethanol under similar conditions gave only pyrazole-5-carboxylic acid, which formed as a result of oxidation followed by decarbonylation. 2-(Pyrazol-3-yl)ethanol in this oxidation reaction gave a mixture of 2-oxo-2-(pyrazol-3-yl)acetic acid and pyrazole-3-carboxylic acid.