Inorg. Chem. 2015, 54 (11), 5169-5181
DOI: 10.1021/ic503061z
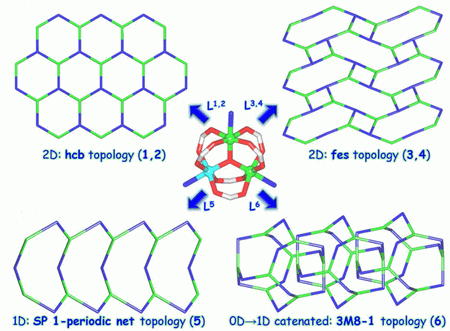
Linkage of the trigonal complex [Fe2NiO(Piv)6] (where Piv- = pivalate) by a series of polypyridine ligands, namely, tris(4-pyridyl)triazine (L2), 2,6-bis(3-pyridyl)-4-(4-pyridyl)pyridine (L3), N-(bis-2,2-(4-pyridyloxymethyl)-3-(4-pyridyloxy)propyl))pyridone-4 (L4), and 4-(N,N-diethylamino)phenyl-bis-2,6-(4-pyridyl)pyridine (L5) resulted in the formation of novel coordination polymers [Fe2NiO(Piv)6(L2)]n (2), [Fe2NiO(Piv)6(L3)]n (3), [Fe2NiO(Piv)6(L4)]n•nHPiv (4), and [{Fe2NiO(Piv)6}4{L5}6]n•3nDEF (5, where DEF is N,N-diethylformamide), which were crystallographically characterized. The topological analysis of 3, 4, and 5 disclosed the 3,3,4,4-connected 2D (3, 4) or 3,4,4-connected 1D (5) underlying networks which, upon further simplification, gave rise to the uninodal 3-connected nets with the respective fes (3, 4) or SP 1-periodic net (4,4)(0,2) (5) topologies, driven by the cluster [Fe2Ni(μ3-O)(μ-Piv)6] nodes and the polypyridine μ3-L3,4 or μ2-L5 blocks. The obtained topologies were compared with those identified in other closely related derivatives [Fe2NiO(Piv)6(L1)]n (1) and {Fe2NiO(Piv)6}8{L6}12 (6), where L1 and L6 are tris(4-pyridyl)pyridine and 4-(N,N-dimethylamino)phenyl-bis-2,6-(4-pyridyl)pyridine, respectively. It was shown that a key structure-driven role in defining the dimensionality and topology of the resulting coordination network is played by the type of polypyridine spacer. Compounds 2 and 3 possess a porous structure, as confirmed by the N2 and H2 sorption data at 78 K. Methanol and ethanol sorption by 2 was also studied indicating that the pores filled by these substrates did not induce any structural rearrangement of this sorbent. Additionally, porous coordination polymer 2 was also applied as a heterogeneous catalyst for the condensation of salicylaldehyde or 9-anthracenecarbaldehyde with malononitrile. The best activity of 2 was observed in the case of salicylaldehyde substrate, resulting in up to 88% conversion into 2-imino-2H-chromen-3-carbonitrile.