Synthesis , 2010, 15, 2588-2598
DOI: 10.1055/s-0029-1218840
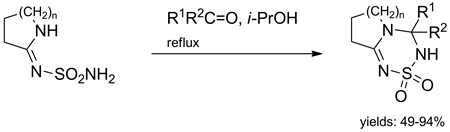
The reactions of five-, six-, seven-, and eight-membered cyclic imido ethers with one equivalent of sulfamide lead to the corresponding sulfamoylamidines in 70-80% yields. Sulfamoylamidines undergo condensations with aliphatic and aromatic aldehydes as well as aliphatic ketones to give thiatriazine dioxides in 50-95% yields. The NMR spectroscopic studies reveal ring-chain tautomerism of some thiatriazine dioxides in solution. The ratio between the tautomers depends on the temperature, solvent polarity, and electronic properties of the substituents of the pendant aryl rings. Thiatriazine dioxides are readily alkylated and acylated at position 2.
Khodachenko A. N.; Shivanyuk A. N.; Shishkina S. V.; Shishkin O. V.; Nazarenko K. G.; Tolmachev A. A.
Synthesis 2010, 15, 2588-2598
DOI: 10.1055/s-0029-1218840
