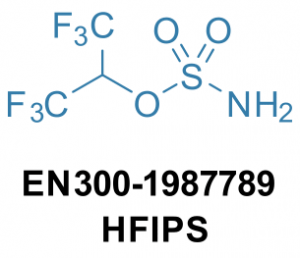
Kyiv, Ukraine, 22 November 2021. Recently, Prof. Magolan and coworkers from McMaster University (Hamilton, Ontario, Canada) reported that hexafluoroisopropyl sulfamate (HFIPS) is a convenient reagent for the sulfamoylation of alcohols and amines (Org. Lett. 2021, 23, 3373–3378). HFIPS has also been used for metal-catalyzed C–H aminations (J. Am. Chem. Soc. 2014, 136, 5783–5789) and for the synthesis of aziridines using silver catalysis (Synthesis 2018, 50, 4462–4470) or electrochemical conditions (Angew. Chem. Int. Ed. 2018, 57, 5695–5698). Enamine has adapted and scaled-up the published procedure to synthesize HFIPS and make it more accessible to scientists worldwide. The reagent is now available in Enamine’s catalogue in multigram quantities.
Iryna Iavniuk, Business Development Director at Enamine, comments: “We at Enamine are committed to making novel reagents immediately accessible to our customers. We have successfully applied the HFIPS reagent in one of our projects previously and were happy to produce it for commercialization.”
Prof. Dr. Oleksandr Grygorenko, Consulting Scientist at Enamine, explains: “Dr. Jarrod Johnson, a Research Associate in the Magolan group, contacted us to point out that there was a supply gap with HFIPS. We recognized the usefulness of the reagent and happily agreed to begin commercialization. Our chemists quickly reproduced the published procedure to synthesize 50 grams of the reagent and HFIPS is now available from EnamineStore: https://www.enaminestore.com/catalog/EN300-1987789.”
Dr. Jarrod Johnson from McMaster University, adds: “We like the ease and simplicity of using HFIPS for sulfamoylation chemistry and hope it might become the go-to reagent for medicinal chemists making sulfamates and sulfamides.”
Prof. Dr. Jakob Magolan from McMaster University, comments: “It’s great to see Enamine now offering bulk HFIPS. I expect that this will enable creative scientists around the world to further extend the scope and applicability of this useful little reagent.”
Conflict of interest statement: Hereby the parties confirm that Prof. Magolan and his group have no financial interest in commercialization of EN300-1987789. No specialized data was transferred to Enamine apart from that already published in the literature.